Real-world effectiveness of systemic agents approved for advanced non-small cell lung cancer: a SEER-Medicare analysis
- PMID: 23635558
- PMCID: PMC3662852
- DOI: 10.1634/theoncologist.2012-0480
Real-world effectiveness of systemic agents approved for advanced non-small cell lung cancer: a SEER-Medicare analysis
Abstract
Objectives: Disparity exists between patients with lung cancer enrolled in clinical trials and patients treated in the community setting. This study assessed the real-world effectiveness of cytotoxic agents that became available for the treatment of non-small cell lung cancer (NSCLC) in the last 2 decades.
Methods: We employed the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database for patients diagnosed with stage IIIB/IV NSCLC between 1988 and 2005 to assess the effectiveness of newly approved agents. Effectiveness of specific agents was assessed at time periods immediately following the approval of the agent for NSCLC: baseline, 1988-1994; platinum, 1995-1999; docetaxel, 1999-2003; pemetrexed and bevacizumab, 2004-2005. Significant associations between specific drug treatment and survival improvement were determined using the Kaplan-Meier method, Cox proportional hazard model, and propensity score analyses. Significant differences were established by log-rank test.
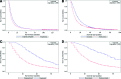
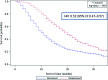
Results: This analysis employed data from 143,548 patients by sex (58% male, 42% female), cancer stage (35% stage IIIB, 65% stage IV), and age (12% 20-64 years, 22% 65-69 years, 45% 70-79 years, 22% 80 years and older). There was temporal improvement in survival for patients treated with newly approved chemotherapy (1-year survival rates: 32.41% in 1988-1994, 32.95% in 1995-1998, 37.40% in 1999-2003, and 39.55% in 2004-2005). Patients treated with a newly approved drug during the relevant treatment era had a significant reduction in the risk of death when compared with patients treated with chemotherapy other than the newly approved agent (hazard ratios [95% confidence interval] were 0.76 [0.71-0.81] for platinum, 0.73 [0.70-0.75] for docetaxel, 0.40 [0.37-0.44] for pemetrexed, and 0.33 [0.27-0.40] for bevacizumab; p < .001). Propensity score adjustment did not significantly alter these results.
Conclusions: Currently approved drugs for the treatment of advanced NSCLC are associated with improved survival in the U.S. Medicare patient population. Our findings support the effectiveness of these agents in the real-world oncology practice.
Keywords: Chemotherapy; Effectiveness; Lung cancer; Real world; SEER–Medicare; Survival.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. - PubMed
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA: Cancer J Clin. 2010;60:277–300. - PubMed
-
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. - PubMed
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

