Weekly paclitaxel/carboplatin/trastuzumab therapy improves pathologic complete remission in aggressive HER2-positive breast cancers, especially in luminal-B subtype, compared with a once-every-3-weeks schedule
- PMID: 23635560
- PMCID: PMC3662841
- DOI: 10.1634/theoncologist.2012-0057
Weekly paclitaxel/carboplatin/trastuzumab therapy improves pathologic complete remission in aggressive HER2-positive breast cancers, especially in luminal-B subtype, compared with a once-every-3-weeks schedule
Abstract
Background: The efficacy and tolerability of two different schedules of paclitaxel, carboplatin, and trastuzumab (PCarH) for HER2-positive, locally aggressive (stage IIB-IIIC) breast cancers were evaluated in this phase II trial.
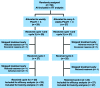
Methods: Patients were randomly assigned to receive either weekly (12 doses over 16 weeks) or once-every-3-weeks (4 doses over 12 weeks) treatment. The primary endpoint was pathologic complete remission (pCR) in the breast and axilla. To detect an assumed 35% pCR absolute difference between the two schedules, a minimum of 26 assessable patients in each group was required (two-sided α = 0.05, β = 0.2).
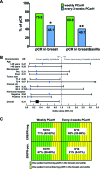
Results: A total of 56 patients were enrolled (weekly group, n = 29; every-3-weeks group, n = 27). In the intent-to-treat analysis, pCR in the breast/axilla were found in 31 patients (55%; 95% confidence interval [CI]: 41%-69%). Compared with the every-3-weeks schedule, the weekly administration achieved higher pCR (41% vs. 69%; p = .03). After adjustment for clinical and pathological factors, the weekly administration was more effective than the every-3-weeks schedule, with hazard ratio of 0.3 (95% CI: 0.1-0.9; p = .03). Interestingly, weekly administration resulted in high pCR rates in both luminal-B (HER2-positive) and ERBB2+ tumors (67% vs. 71%; p = .78), whereas luminal-B (HER2-positive) tumors benefited less from the every-3-weeks schedule compared with the ERBB2+ tumors (21% vs. 62%, p = .03). These results remain after multivariate adjustment, showing weekly administration was more effective in the luminal-B (HER2-positive) subgroup (p = .02) but not in the ERBB2+ subgroup (p = .50).
Conclusion: A more frequent administration might improve the possibility of eradicating invasive cancer in the breast and axilla, especially in the luminal-B (HER2-positive) subtype. Further studies to validate our findings are warranted.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. - PubMed
-
- Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: Results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. - PubMed
-
- Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

