Complications and continuation of intrauterine device use among commercially insured teenagers
- PMID: 23635730
- PMCID: PMC4028832
- DOI: 10.1097/AOG.0b013e31828b63a0
Complications and continuation of intrauterine device use among commercially insured teenagers
Abstract
Objective: Many U.S. health care providers remain reluctant to prescribe intrauterine devices (IUDs) to teenagers as a result of concerns about serious complications. This study examined whether 15-19-year-old IUD users were more likely to experience complications, failure, or early discontinuation than adult users aged 20-24 years and 25-44 years and whether there were differences in these outcomes between users of levonorgestrel-releasing intrauterine systems and copper IUDs.
Methods: A retrospective cohort study was conducted using health insurance claims obtained from a private insurance company of 90,489 women who had an IUD inserted between 2002 and 2009. Logistic regression models were used to estimate the odds of experiencing complications, method failure, or early discontinuation within 12 months of insertion by age group and type of IUD inserted.
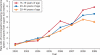
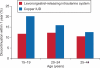
Results: Serious complications, including ectopic pregnancy and pelvic inflammatory disease, occurred in less than 1% of patients regardless of age or IUD type. Women aged 15-19 years were more likely than those aged 25-44 years to have a claim for dysmenorrhea (odds ratio [OR] 1.4, confidence interval [CI] 1.1-1.6), amenorrhea (OR 1.3, CI 1.1-1.5), or normal pregnancy (OR 1.4, CI 1.1-1.8). Overall, early discontinuation did not differ between teenagers and women aged 25-44 years (13% compared with 11%, P>.05). However, use of the levonorgestrel-releasing intrauterine system was associated with fewer complications and less early discontinuation than the copper IUD in all age groups.
Conclusions: The IUD is as appropriate for teenagers to use as it is for older women, with serious complications occurring infrequently in all groups. The levonorgestrel-releasing intrauterine system may be a better choice than the copper IUD as a result of lower odds of complications, discontinuation, and failure.
Level of evidence: II.
Figures
Similar articles
-
Safety of levonorgestrel 52 mg intrauterine system compared to copper intrauterine device: a population-based cohort study.Contraception. 2019 Jun;99(6):345-349. doi: 10.1016/j.contraception.2019.02.011. Epub 2019 Mar 12. Contraception. 2019. PMID: 30871933
-
Effects of age, parity, and device type on complications and discontinuation of intrauterine devices.Obstet Gynecol. 2014 Mar;123(3):585-592. doi: 10.1097/AOG.0000000000000144. Obstet Gynecol. 2014. PMID: 24499755
-
Free-of-charge long-acting reversible contraception: two-year discontinuation, its risk factors, and reasons.Am J Obstet Gynecol. 2020 Dec;223(6):886.e1-886.e17. doi: 10.1016/j.ajog.2020.06.023. Epub 2020 Jun 17. Am J Obstet Gynecol. 2020. PMID: 32562657
-
An evaluation of the levonorgestrel-releasing IUD: its advantages and disadvantages when compared to the copper-releasing IUDs.Contraception. 1991 Dec;44(6):573-88. doi: 10.1016/0010-7824(91)90078-t. Contraception. 1991. PMID: 1773615 Review.
-
Levonorgestrel-releasing IUD as a method of contraception with therapeutic properties.Contraception. 1995 Nov;52(5):269-76. doi: 10.1016/0010-7824(95)00210-2. Contraception. 1995. PMID: 8585882 Review.
Cited by
-
Use of frameless intrauterine devices and systems in young nulliparous and adolescent women: results of a multicenter study.Int J Womens Health. 2014 Aug 6;6:727-34. doi: 10.2147/IJWH.S65462. eCollection 2014. Int J Womens Health. 2014. PMID: 25125987 Free PMC article.
-
Long-Term Retained Lippes Loop Intrauterine Device Causes Vesicouterine Fistula.Cureus. 2023 Apr 27;15(4):e38217. doi: 10.7759/cureus.38217. eCollection 2023 Apr. Cureus. 2023. PMID: 37252518 Free PMC article.
-
The safety of intrauterine devices among young women: a systematic review.Contraception. 2017 Jan;95(1):17-39. doi: 10.1016/j.contraception.2016.10.006. Epub 2016 Oct 19. Contraception. 2017. PMID: 27771475 Free PMC article.
-
Profiles of copper intrauterine devices and levonorgestrel intrauterine systems users in France in 2019: A national observational population-based study.Int J Gynaecol Obstet. 2023 Feb;160(2):594-603. doi: 10.1002/ijgo.14438. Epub 2022 Sep 10. Int J Gynaecol Obstet. 2023. PMID: 36066002 Free PMC article.
-
Intrauterine contraception in nulliparous women: a prospective survey.J Fam Plann Reprod Health Care. 2016 Jan;42(1):36-42. doi: 10.1136/jfprhc-2014-101046. Epub 2015 Apr 8. J Fam Plann Reprod Health Care. 2016. PMID: 25854550 Free PMC article.
References
-
- Sivin I, Stern J, Coutinho E, Mattos CER, Mahgoub SE, Diaz S, et al. Prolonged intrauterine contraception: a seven-year randomized study of the Levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDs. Contraception. 1991;44(5):473–80. - PubMed
-
- World Health Organization . Medical eligibility criteria for contraceptive use. 3rd ed. Geneva, Switzerland: 2004.
-
- ACOG Committee Opinion Intrauterine device and adolescents. Obstet Gynecol. 2007;110(6):1493–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

