Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses
- PMID: 23635775
- PMCID: PMC3668833
- DOI: 10.1172/JCI60945
Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses
Abstract
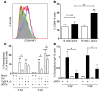
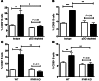
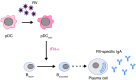
B cell-dependent immunity to rotavirus, an important intestinal pathogen, plays a significant role in viral clearance and protects against reinfection. Human in vitro and murine in vivo models of rotavirus infection were used to delineate the role of primary plasmacytoid DCs (pDCs) in initiating B cell responses. Human pDCs were necessary and sufficient for B cell activation induced by rotavirus. Type I IFN recognition by B cells was essential for rotavirus-mediated B cell activation in vitro and murine pDCs and IFN-α/β-mediated B cell activation after in vivo intestinal rotavirus infection. Furthermore, rotavirus-specific serum and mucosal antibody responses were defective in mice lacking functional pDCs at the time of infection. These data demonstrate that optimal B cell activation and virus-specific antibody secretion following mucosal infection were a direct result of pDC-derived type I IFN. Importantly, viral shedding significantly increased in pDC-deficient mice, suggesting that pDC-dependent antibody production influences viral clearance. Thus, mucosal pDCs critically influence the course of rotavirus infection through rotavirus recognition and subsequent IFN production and display powerful adjuvant properties to initiate and enhance humoral immunity.
Figures





Similar articles
-
Rotavirus structural proteins and dsRNA are required for the human primary plasmacytoid dendritic cell IFNalpha response.PLoS Pathog. 2010 Jun 3;6(6):e1000931. doi: 10.1371/journal.ppat.1000931. PLoS Pathog. 2010. PMID: 20532161 Free PMC article.
-
Innate immune responses to human rotavirus in the neonatal gnotobiotic piglet disease model.Immunology. 2010 Oct;131(2):242-56. doi: 10.1111/j.1365-2567.2010.03298.x. Immunology. 2010. PMID: 20497255 Free PMC article.
-
Dendritic cell expression of MyD88 is required for rotavirus-induced B cell activation.J Virol. 2025 May 20;99(5):e0065325. doi: 10.1128/jvi.00653-25. Epub 2025 Apr 30. J Virol. 2025. PMID: 40304491 Free PMC article.
-
Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance.Immunol Rev. 2010 Mar;234(1):142-62. doi: 10.1111/j.0105-2896.2009.00881.x. Immunol Rev. 2010. PMID: 20193017 Free PMC article. Review.
-
Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat.Front Immunol. 2019 Apr 12;10:778. doi: 10.3389/fimmu.2019.00778. eCollection 2019. Front Immunol. 2019. PMID: 31031767 Free PMC article. Review.
Cited by
-
Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections.PLoS Pathog. 2016 Apr 29;12(4):e1005600. doi: 10.1371/journal.ppat.1005600. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27128797 Free PMC article.
-
The dark side of the gut: Virome-host interactions in intestinal homeostasis and disease.J Exp Med. 2021 May 3;218(5):e20201044. doi: 10.1084/jem.20201044. J Exp Med. 2021. PMID: 33760921 Free PMC article. Review.
-
Impact of BAFF Blockade on Inflammation, Germinal Center Reaction and Effector B-Cells During Acute SIV Infection.Front Immunol. 2020 Feb 28;11:252. doi: 10.3389/fimmu.2020.00252. eCollection 2020. Front Immunol. 2020. PMID: 32194549 Free PMC article.
-
Treading a HOSTile path: Mapping the dynamic landscape of host cell-rotavirus interactions to explore novel host-directed curative dimensions.Virulence. 2021 Dec;12(1):1022-1062. doi: 10.1080/21505594.2021.1903198. Virulence. 2021. PMID: 33818275 Free PMC article. Review.
-
Proteomic and Single-Cell Transcriptomic Dissection of Human Plasmacytoid Dendritic Cell Response to Influenza Virus.Front Immunol. 2022 Mar 23;13:814627. doi: 10.3389/fimmu.2022.814627. eCollection 2022. Front Immunol. 2022. PMID: 35401570 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

