Efficacious proteasome/HDAC inhibitor combination therapy for primary effusion lymphoma
- PMID: 23635777
- PMCID: PMC3668825
- DOI: 10.1172/JCI64503
Efficacious proteasome/HDAC inhibitor combination therapy for primary effusion lymphoma
Abstract
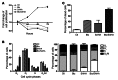
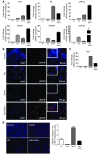
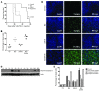
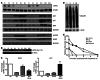
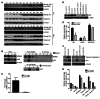
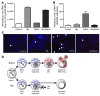
Primary effusion lymphoma (PEL) is a rare form of aggressive B cell lymphoma caused by Kaposi's sarcoma-associated herpesvirus (KSHV). Current chemotherapy approaches result in dismal outcomes, and there is an urgent need for new PEL therapies. Previously, we established, in a direct xenograft model of PEL-bearing immune-compromised mice, that treatment with the proteasome inhibitor, bortezomib (Btz), increased survival relative to that after treatment with doxorubicin. Herein, we demonstrate that the combination of Btz with the histone deacetylase (HDAC) inhibitor suberoylanilidehydroxamic acid (SAHA, also known as vorinostat) potently reactivates KSHV lytic replication and induces PEL cell death, resulting in significantly prolonged survival of PEL-bearing mice. Importantly, Btz blocked KSHV late lytic gene expression, terminally inhibiting the full lytic cascade and production of infectious virus in vivo. Btz treatment led to caspase activation and induced DNA damage, as evidenced by the accumulation of phosphorylated γH2AX and p53. The addition of SAHA to Btz treatment was synergistic, as SAHA induced early acetylation of p53 and reduced interaction with its negative regulator MDM2, augmenting the effects of Btz. The eradication of KSHV-infected PEL cells without increased viremia in mice provides a strong rationale for using the proteasome/HDAC inhibitor combination therapy in PEL.
Figures

Similar articles
-
Repurposing Cytarabine for Treating Primary Effusion Lymphoma by Targeting Kaposi's Sarcoma-Associated Herpesvirus Latent and Lytic Replications.mBio. 2018 May 8;9(3):e00756-18. doi: 10.1128/mBio.00756-18. mBio. 2018. PMID: 29739902 Free PMC article.
-
Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma.Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13069-74. doi: 10.1073/pnas.1002985107. Epub 2010 Jul 6. Proc Natl Acad Sci U S A. 2010. PMID: 20615981 Free PMC article.
-
EZH2 Inhibition by DS3201 Triggers the Kaposi's Sarcoma-Associated Herpesvirus Lytic Cycle and Potentiates the Effects Induced by SAHA in Primary Effusion Lymphoma Cells.Viruses. 2024 Sep 20;16(9):1490. doi: 10.3390/v16091490. Viruses. 2024. PMID: 39339966 Free PMC article.
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
-
Primary effusion lymphoma: therapeutic strategies targeting viral and cellular mechanisms.Expert Rev Anticancer Ther. 2025 Apr;25(4):363-381. doi: 10.1080/14737140.2025.2474728. Epub 2025 Mar 6. Expert Rev Anticancer Ther. 2025. PMID: 40033827 Review.
Cited by
-
Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy.J Clin Invest. 2016 Sep 1;126(9):3165-75. doi: 10.1172/JCI84418. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584730 Free PMC article. Review.
-
Targeting Host Cellular Factors as a Strategy of Therapeutic Intervention for Herpesvirus Infections.Front Cell Infect Microbiol. 2021 Mar 19;11:603309. doi: 10.3389/fcimb.2021.603309. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816328 Free PMC article. Review.
-
Sirtuin 6 Attenuates Kaposi's Sarcoma-Associated Herpesvirus Reactivation by Suppressing Ori-Lyt Activity and Expression of RTA.J Virol. 2019 Mar 21;93(7):e02200-18. doi: 10.1128/JVI.02200-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651359 Free PMC article.
-
Impact of Myc in HIV-associated non-Hodgkin lymphomas treated with EPOCH and outcomes with vorinostat (AMC-075 trial).Blood. 2020 Sep 10;136(11):1284-1297. doi: 10.1182/blood.2019003959. Blood. 2020. PMID: 32430507 Free PMC article. Clinical Trial.
-
Repression of varicella zoster virus gene expression during quiescent infection in the absence of detectable histone deposition.PLoS Pathog. 2025 Feb 10;21(2):e1012367. doi: 10.1371/journal.ppat.1012367. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39928684 Free PMC article.
References
-
- Cesarman E, Mesri EA. Pathogenesis of viral lymphomas. Cancer Treat Res. 2006;131:49–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA112217/CA/NCI NIH HHS/United States
- R01 CA136387/CA/NCI NIH HHS/United States
- CA136387/CA/NCI NIH HHS/United States
- NCI2U01CA121947-04/CA/NCI NIH HHS/United States
- L30 CA117648/CA/NCI NIH HHS/United States
- 5P30AI073961-05/AI/NIAID NIH HHS/United States
- CA122105/CA/NCI NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- P01-CA-128115-01A2/CA/NCI NIH HHS/United States
- P01 CA128115/CA/NCI NIH HHS/United States
- R01 CA109335/CA/NCI NIH HHS/United States
- R01 CA122105/CA/NCI NIH HHS/United States
- CA109335/CA/NCI NIH HHS/United States
- U01 CA121947/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

