Evolving therapies for liver fibrosis
- PMID: 23635787
- PMCID: PMC3635731
- DOI: 10.1172/JCI66028
Evolving therapies for liver fibrosis
Abstract
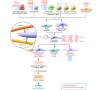
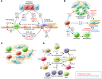
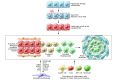
Fibrosis is an intrinsic response to chronic injury, maintaining organ integrity when extensive necrosis or apoptosis occurs. With protracted damage, fibrosis can progress toward excessive scarring and organ failure, as in liver cirrhosis. To date, antifibrotic treatment of fibrosis represents an unconquered area for drug development, with enormous potential but also high risks. Preclinical research has yielded numerous targets for antifibrotic agents, some of which have entered early-phase clinical studies, but progress has been hampered due to the relative lack of sensitive and specific biomarkers to measure fibrosis progression or reversal. Here we focus on antifibrotic approaches for liver that address specific cell types and functional units that orchestrate fibrotic wound healing responses and have a sound preclinical database or antifibrotic activity in early clinical trials. We also touch upon relevant clinical study endpoints, optimal study design, and developments in fibrosis imaging and biomarkers.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

