Benzofuran-, benzothiophene-, indazole- and benzisoxazole-quinones: excellent substrates for NAD(P)H:quinone oxidoreductase 1
- PMID: 23635904
- PMCID: PMC3697102
- DOI: 10.1016/j.bmc.2013.03.071
Benzofuran-, benzothiophene-, indazole- and benzisoxazole-quinones: excellent substrates for NAD(P)H:quinone oxidoreductase 1
Abstract
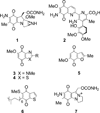
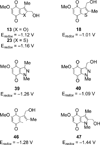
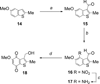
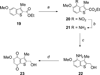
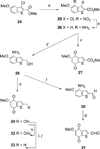
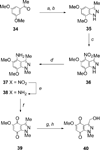
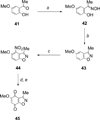
A series of heterocyclic quinones based on benzofuran, benzothiophene, indazole and benzisoxazole has been synthesized, and evaluated for their ability to function as substrates for recombinant human NAD(P)H:quinone oxidoreductase (NQO1), a two-electron reductase upregulated in tumor cells. Overall, the quinones are excellent substrates for NQO1, approaching the reduction rates observed for menadione.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1: from synthetic organic chemistry to compounds with anticancer potential.Org Biomol Chem. 2008 Feb 21;6(4):637-56. doi: 10.1039/b715270a. Epub 2007 Dec 13. Org Biomol Chem. 2008. PMID: 18264564 Review.
-
Novel quinolinequinone antitumor agents: structure-metabolism studies with NAD(P)H:quinone oxidoreductase (NQO1).Bioorg Med Chem. 2004 Apr 1;12(7):1667-87. doi: 10.1016/j.bmc.2004.01.021. Bioorg Med Chem. 2004. PMID: 15028260
-
Benzimidazole- and benzothiazole-quinones: excellent substrates for NAD(P)H:quinone oxidoreductase 1.Org Biomol Chem. 2007 Nov 21;5(22):3665-73. doi: 10.1039/b713044a. Epub 2007 Oct 8. Org Biomol Chem. 2007. PMID: 17971996
-
Indolequinone antitumor agents: correlation between quinone structure, rate of metabolism by recombinant human NAD(P)H:quinone oxidoreductase, and in vitro cytotoxicity.J Med Chem. 1998 Nov 19;41(24):4755-66. doi: 10.1021/jm980328r. J Med Chem. 1998. PMID: 9822546
-
NAD(P)H:Quinone Oxidoreductase 1 (NQO1) as a Therapeutic and Diagnostic Target in Cancer.J Med Chem. 2018 Aug 23;61(16):6983-7003. doi: 10.1021/acs.jmedchem.8b00124. Epub 2018 May 7. J Med Chem. 2018. PMID: 29712428 Review.
Cited by
-
Phytonutrients Differentially Stimulate NAD(P)H:Quinone Oxidoreductase, Inhibit Proliferation, and Trigger Mitotic Catastrophe in Hepa1c1c7 Cells.J Med Food. 2016 Jan;19(1):47-53. doi: 10.1089/jmf.2015.0079. Epub 2015 Dec 1. J Med Food. 2016. PMID: 26623679 Free PMC article.
References
-
- Thomson RH. Naturally Occurring Quinones IV. Recent advances. 4th ed. London: Blackie; 1997.
-
- Tisler M. Adv. Heterocycl. Chem. 1989;45:37–150.
-
- Boutros R, Lobjois V, Ducommun B. Nat. Rev. Cancer. 2007;7:495–507. - PubMed
-
- Carter SK, Crooke ST. Mitomycin C; Current Status and New Developments. New York: Academic Press; 1979.
-
- Remers WA, Dorr RT. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. New York: Wiley; 1988. pp. 1–74.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

