Transduction of voltage and Ca2+ signals by Slo1 BK channels
- PMID: 23636263
- PMCID: PMC3742125
- DOI: 10.1152/physiol.00055.2012
Transduction of voltage and Ca2+ signals by Slo1 BK channels
Abstract
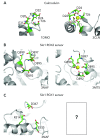
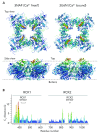
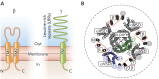
Large-conductance Ca2+ -and voltage-gated K+ channels are activated by an increase in intracellular Ca2+ concentration and/or depolarization. The channel activation mechanism is well described by an allosteric model encompassing the gate, voltage sensors, and Ca2+ sensors, and the model is an excellent framework to understand the influences of auxiliary β and γ subunits and regulatory factors such as Mg2+. Recent advances permit elucidation of structural correlates of the biophysical mechanism.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures

References
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9: 209–216, 1992 - PubMed
-
- Altura BM, Altura BT, Gupta RK. Alcohol intoxication results in rapid loss in free magnesium in brain and disturbances in brain bioenergetics: relation to cerebrovasospasm, alcohol-induced strokes, and barbiturate anesthesia-induced deaths. Magnes Trace Elem 10: 122–135, 1991 - PubMed
-
- Altura BM, Zhang A, Cheng TP, Altura BT. Ethanol promotes rapid depletion of intracellular free Mg in cerebral vascular smooth muscle cells: possible relation to alcohol-induced behavioral and stroke-like effects. Alcohol 10: 563–566, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

