Structure of the human smoothened receptor bound to an antitumour agent
- PMID: 23636324
- PMCID: PMC3657389
- DOI: 10.1038/nature12167
Structure of the human smoothened receptor bound to an antitumour agent
Abstract
The smoothened (SMO) receptor, a key signal transducer in the hedgehog signalling pathway, is responsible for the maintenance of normal embryonic development and is implicated in carcinogenesis. It is classified as a class frizzled (class F) G-protein-coupled receptor (GPCR), although the canonical hedgehog signalling pathway involves the GLI transcription factors and the sequence similarity with class A GPCRs is less than 10%. Here we report the crystal structure of the transmembrane domain of the human SMO receptor bound to the small-molecule antagonist LY2940680 at 2.5 Å resolution. Although the SMO receptor shares the seven-transmembrane helical fold, most of the conserved motifs for class A GPCRs are absent, and the structure reveals an unusually complex arrangement of long extracellular loops stabilized by four disulphide bonds. The ligand binds at the extracellular end of the seven-transmembrane-helix bundle and forms extensive contacts with the loops.
Figures
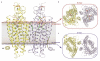
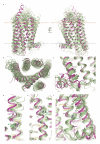
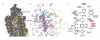
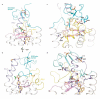

Comment in
-
G protein-coupled receptors: pioneering Frizzled family receptor structure solved.Nat Rev Drug Discov. 2013 Jun;12(6):424. doi: 10.1038/nrd4030. Epub 2013 May 17. Nat Rev Drug Discov. 2013. PMID: 23681005 No abstract available.
References
-
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi:10.1101/gad.938601. - PubMed
-
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi:10.1038/384176a0. - PubMed
-
- Stone DM, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi:10.1038/384129a0. - PubMed
-
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi:10.1038/nature00989. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

