NLRP3 deletion protects from hyperoxia-induced acute lung injury
- PMID: 23636457
- PMCID: PMC3725631
- DOI: 10.1152/ajpcell.00086.2013
NLRP3 deletion protects from hyperoxia-induced acute lung injury
Abstract
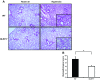
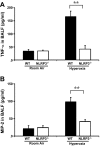
Inspiration of a high concentration of oxygen, a therapy for acute lung injury (ALI), could unexpectedly lead to reactive oxygen species (ROS) production and hyperoxia-induced acute lung injury (HALI). Nucleotide-binding domain and leucine-rich repeat PYD-containing protein 3 (NLRP3) senses the ROS, triggering inflammasome activation and interleukin-1β (IL-1β) production and secretion. However, the role of NLRP3 inflammasome in HALI is unclear. The main aim of this study is to determine the effect of NLRP3 gene deletion on inflammatory response and lung epithelial cell death. Wild-type (WT) and NLRP3(-/-) mice were exposed to 100% O2 for 48-72 h. Bronchoalveolar lavage fluid and lung tissues were examined for proinflammatory cytokine production and lung inflammation. Hyperoxia-induced lung pathological score was suppressed in NLRP3(-/-) mice compared with WT mice. Hyperoxia-induced recruitment of inflammatory cells and elevation of IL-1β, TNFα, macrophage inflammatory protein-2, and monocyte chemoattractant protein-1 were attenuated in NLRP3(-/-) mice. NLRP3 deletion decreased lung epithelial cell death and caspase-3 levels and a suppressed NF-κB levels compared with WT controls. Taken together, this research demonstrates for the first time that NLRP3-deficient mice have suppressed inflammatory response and blunted lung epithelial cell apoptosis to HALI.
Keywords: hyperoxia; inflammation; injury; lung; reactive oxygen species.
Figures
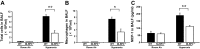



Similar articles
-
CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury.Inflamm Res. 2016 Nov;65(11):905-915. doi: 10.1007/s00011-016-0973-7. Epub 2016 Jul 13. Inflamm Res. 2016. PMID: 27412237
-
Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression.Am J Physiol Lung Cell Mol Physiol. 2016 Mar 15;310(6):L572-81. doi: 10.1152/ajplung.00417.2015. Epub 2016 Jan 8. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26747786 Free PMC article.
-
NLRP3 protein deficiency exacerbates hyperoxia-induced lethality through Stat3 protein signaling independent of interleukin-1β.J Biol Chem. 2015 Feb 20;290(8):5065-5077. doi: 10.1074/jbc.M114.603217. Epub 2014 Dec 29. J Biol Chem. 2015. PMID: 25548278 Free PMC article.
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
-
Hyperoxic acute lung injury.Respir Care. 2013 Jan;58(1):123-41. doi: 10.4187/respcare.01963. Respir Care. 2013. PMID: 23271823 Free PMC article. Review.
Cited by
-
NOD2-NLRP3 Axis and Asthma.J Asthma Allergy. 2025 May 22;18:769-777. doi: 10.2147/JAA.S526788. eCollection 2025. J Asthma Allergy. 2025. PMID: 40433101 Free PMC article. Review.
-
NLRP3 Regulated CXCL12 Expression in Acute Neutrophilic Lung Injury.J Inflamm Res. 2020 Jul 23;13:377-386. doi: 10.2147/JIR.S259633. eCollection 2020. J Inflamm Res. 2020. PMID: 32801831 Free PMC article.
-
CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury.Inflamm Res. 2016 Nov;65(11):905-915. doi: 10.1007/s00011-016-0973-7. Epub 2016 Jul 13. Inflamm Res. 2016. PMID: 27412237
-
Mitochondrial Protein Akap1 Deletion Exacerbates Endoplasmic Reticulum Stress in Mice Exposed to Hyperoxia.Front Pharmacol. 2022 Mar 14;13:762840. doi: 10.3389/fphar.2022.762840. eCollection 2022. Front Pharmacol. 2022. PMID: 35370705 Free PMC article.
-
Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation.Sci Rep. 2016 Dec 22;6:39473. doi: 10.1038/srep39473. Sci Rep. 2016. PMID: 28004759 Free PMC article.
References
-
- Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 279: L1137–L1145, 2000 - PubMed
-
- Bethea JR, Chung IY, Sparacio SM, Gillespie GY, Benveniste EN. Interleukin-1 beta induction of tumor necrosis factor-alpha gene expression in human astroglioma cells. J Neuroimmunol 36: 179–191, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

