Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings
- PMID: 23636718
- PMCID: PMC3727063
- DOI: 10.1152/jn.01104.2012
Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings
Abstract
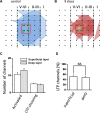
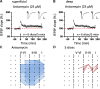
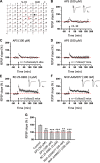
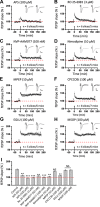
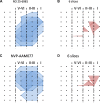
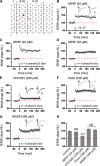
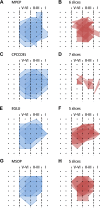
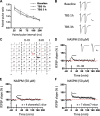
The insular cortex (IC) is widely believed to be an important forebrain structure involved in cognitive and sensory processes such as memory and pain. However, little work has been performed at the cellular level to investigate the synaptic basis of IC-related brain functions. To bridge the gap, the present study was designed to characterize the basic synaptic mechanisms for insular long-term potentiation (LTP). Using a 64-channel recording system, we found that an enduring form of late-phase LTP (L-LTP) could be reliably recorded for at least 3 h in different layers of IC slices after theta burst stimulation. The induction of insular LTP is protein synthesis dependent and requires activation of both GluN2A and GluN2B subunits of the NMDA receptor, L-type voltage-gated calcium channels, and metabotropic glutamate receptor 1. The paired-pulse facilitation ratio was unaffected by insular L-LTP induction, and expression of insular L-LTP required the recruitment of postsynaptic calcium-permeable AMPA receptors. Our results provide the first in vitro report of long-term multichannel recordings of L-LTP in the IC in adult mice and suggest its potential important roles in insula-related memory and chronic pain.
Keywords: calcium-permeable AMPA receptor; glutamate receptor; insular cortex; long-term potentiation; multielectrode array.
Figures




Similar articles
-
Long-term depression of synaptic transmission in the adult mouse insular cortex in vitro.Eur J Neurosci. 2013 Oct;38(8):3128-45. doi: 10.1111/ejn.12330. Epub 2013 Aug 11. Eur J Neurosci. 2013. PMID: 23930740
-
Essential roles of mGluR1 and inhibitory synaptic transmission in NMDA-independent long-term potentiation in the spinal trigeminal interpolaris.Life Sci. 2016 Jan 1;144:54-60. doi: 10.1016/j.lfs.2015.11.024. Epub 2015 Nov 24. Life Sci. 2016. PMID: 26620765
-
Metabotropic glutamate receptors are involved in calcium-induced LTP of AMPA and NMDA receptor-mediated responses in the rat hippocampus.Brain Res Bull. 1998 Aug;46(6):505-12. doi: 10.1016/s0361-9230(98)00046-x. Brain Res Bull. 1998. PMID: 9744287
-
Memory and the brain: unexpected chemistries and a new pharmacology.Neurobiol Learn Mem. 1998 Jul-Sep;70(1-2):82-100. doi: 10.1006/nlme.1998.3840. Neurobiol Learn Mem. 1998. PMID: 9753589 Review.
-
[Advancement in mechanisms of long-term potentiation].Sheng Li Ke Xue Jin Zhan. 2001 Oct;32(4):298-301. Sheng Li Ke Xue Jin Zhan. 2001. PMID: 12545854 Review. Chinese.
Cited by
-
Multiple synaptic connections into a single cortical pyramidal cell or interneuron in the anterior cingulate cortex of adult mice.Mol Brain. 2021 Jun 3;14(1):88. doi: 10.1186/s13041-021-00793-8. Mol Brain. 2021. PMID: 34082805 Free PMC article.
-
Presynaptic long-term potentiation requires extracellular signal-regulated kinases in the anterior cingulate cortex.Mol Pain. 2020 Jan-Dec;16:1744806920917245. doi: 10.1177/1744806920917245. Mol Pain. 2020. PMID: 32264746 Free PMC article. Review.
-
Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain.Nat Rev Neurosci. 2016 Aug;17(8):485-96. doi: 10.1038/nrn.2016.68. Epub 2016 Jun 16. Nat Rev Neurosci. 2016. PMID: 27307118 Review.
-
Pharmacological rescue of cortical synaptic and network potentiation in a mouse model for fragile X syndrome.Neuropsychopharmacology. 2014 Jul;39(8):1955-67. doi: 10.1038/npp.2014.44. Epub 2014 Feb 20. Neuropsychopharmacology. 2014. PMID: 24553731 Free PMC article.
-
Recruitment of cortical silent responses by forskolin in the anterior cingulate cortex of adult mice.Mol Pain. 2024 Jan-Dec;20:17448069241258110. doi: 10.1177/17448069241258110. Mol Pain. 2024. PMID: 38744422 Free PMC article.
References
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev 29: 83–120, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

