New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms?
- PMID: 23636773
- PMCID: PMC3796138
- DOI: 10.1007/s00424-013-1284-2
New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms?
Abstract
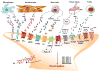
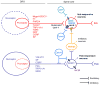
Itch and pain are closely related but distinct sensations. They share largely overlapping mediators and receptors, and itch-responding neurons are also sensitive to pain stimuli. Itch-mediating primary sensory neurons are equipped with distinct receptors and ion channels for itch transduction, including Mas-related G protein-coupled receptors (Mrgprs), protease-activated receptors, histamine receptors, bile acid receptor, toll-like receptors, and transient receptor potential subfamily V1/A1 (TRPV1/A1). Recent progress has indicated the existence of an itch-specific neuronal circuitry. The MrgprA3-expressing primary sensory neurons exclusively innervate the epidermis of skin, and their central axons connect with gastrin-releasing peptide receptor (GRPR)-expressing neurons in the superficial spinal cord. Notably, ablation of MrgprA3-expressing primary sensory neurons or GRPR-expressing spinal cord neurons results in selective reduction in itch but not pain. Chronic itch results from dysfunction of the immune and nervous system and can manifest as neural plasticity despite the fact that chronic itch is often treated by dermatologists. While differences between acute pain and acute itch are striking, chronic itch and chronic pain share many similar mechanisms, including peripheral sensitization (increased responses of primary sensory neurons to itch and pain mediators), central sensitization (hyperactivity of spinal projection neurons and excitatory interneurons), loss of inhibitory control in the spinal cord, and neuro-immune and neuro-glial interactions. Notably, painful stimuli can elicit itch in some chronic conditions (e.g., atopic dermatitis), and some drugs for treating chronic pain are also effective in chronic itch. Thus, itch and pain have more similarities in pathological and chronic conditions.
Conflict of interest statement
The authors have no financial interest in this study.
Figures

Similar articles
-
Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition.Brain. 2021 Mar 3;144(2):665-681. doi: 10.1093/brain/awaa430. Brain. 2021. PMID: 33367648
-
Chronic itch development in sensory neurons requires BRAF signaling pathways.J Clin Invest. 2013 Nov;123(11):4769-80. doi: 10.1172/JCI70528. J Clin Invest. 2013. PMID: 24216512 Free PMC article.
-
Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out?Clin Rev Allergy Immunol. 2016 Dec;51(3):263-292. doi: 10.1007/s12016-015-8488-5. Clin Rev Allergy Immunol. 2016. PMID: 25931325 Review.
-
A subpopulation of nociceptors specifically linked to itch.Nat Neurosci. 2013 Feb;16(2):174-82. doi: 10.1038/nn.3289. Epub 2012 Dec 23. Nat Neurosci. 2013. PMID: 23263443 Free PMC article.
-
Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms?Pulm Pharmacol Ther. 2015 Dec;35:81-6. doi: 10.1016/j.pupt.2015.09.001. Epub 2015 Sep 6. Pulm Pharmacol Ther. 2015. PMID: 26351759 Free PMC article. Review.
Cited by
-
A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain.Neurobiol Pain. 2024 Jan 20;15:100151. doi: 10.1016/j.ynpai.2024.100151. eCollection 2024 Jan-Jun. Neurobiol Pain. 2024. PMID: 38314104 Free PMC article. Review.
-
Role of ERK1/2 activation on itch sensation induced by bradykinin B1 activation in inflamed skin.Exp Ther Med. 2016 Aug;12(2):627-632. doi: 10.3892/etm.2016.3426. Epub 2016 Jun 6. Exp Ther Med. 2016. PMID: 27446253 Free PMC article.
-
Neuroinflammation Involved in Diabetes-Related Pain and Itch.Front Pharmacol. 2022 Jun 20;13:921612. doi: 10.3389/fphar.2022.921612. eCollection 2022. Front Pharmacol. 2022. PMID: 35795572 Free PMC article. Review.
-
The Neuroimmune Axis in Skin Sensation, Inflammation, and Immunity.J Immunol. 2019 May 15;202(10):2829-2835. doi: 10.4049/jimmunol.1801473. J Immunol. 2019. PMID: 31061146 Free PMC article. Review.
-
The Relationship Between Ocular Itch, Ocular Pain, and Dry Eye Symptoms (An American Ophthalmological Society Thesis).Trans Am Ophthalmol Soc. 2018 Jan 17;115:T5. eCollection 2017 Aug. Trans Am Ophthalmol Soc. 2018. PMID: 29391860 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

