Preparation and evaluation of solid dispersions of a new antitumor compound based on early-stage preparation discovery concept
- PMID: 23636816
- PMCID: PMC3665985
- DOI: 10.1208/s12249-013-9948-y
Preparation and evaluation of solid dispersions of a new antitumor compound based on early-stage preparation discovery concept
Abstract
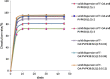
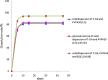
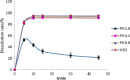
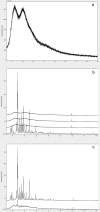
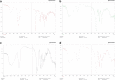
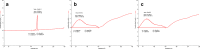
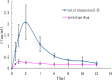
Ensuring sufficient drug solubility is a crucial problem in pharmaceutical-related research. For water-insoluble drugs, various formulation approaches are employed to enhance the solubility and bioavailability of lead compounds. The goal of this study was to enhance the dissolution and absorption of a new antitumor lead compound, T-OA. Early-stage preparation discovery concept was employed in this study. Based on this concept, a solid dispersion system was chosen as the method of improving drug solubility and bioavailability. Solid dispersions of T-OA in polyvinylpyrrolidone (PVP) K30 were prepared by the solvent evaporation method. Dissolution testing determined that the ideal drug-to-PVP ratio was 1:5. X-ray diffraction, Fourier transform infrared spectroscopy, and differential scanning calorimetry were employed to confirm the formation of solid dispersions. Scanning electron microscopy demonstrated that T-OA was converted into an amorphous form. Both in vitro dissolution testing and the in vivo studies demonstrated that the solubility and bioavailability of T-OA were significantly improved when formulated in a solid dispersion with PVP. The dissolution rate of the T-OA/PVP solid dispersion was greatly enhanced relative to the pure drug, and the relative bioavailability of T-OA solid dispersions was found to be 392.0%, which is 4-fold higher than the pure drug.
Figures


Similar articles
-
In-vitro and in-vivo comparison of T-OA microemulsions and solid dispersions based on EPDC.Drug Dev Ind Pharm. 2015 Feb;41(2):263-71. doi: 10.3109/03639045.2013.858739. Epub 2013 Nov 20. Drug Dev Ind Pharm. 2015. PMID: 24256156
-
Preparation, characterization and in vitro/vivo evaluation of tectorigenin solid dispersion with improved dissolution and bioavailability.Eur J Drug Metab Pharmacokinet. 2016 Aug;41(4):413-22. doi: 10.1007/s13318-015-0265-6. Epub 2015 Feb 11. Eur J Drug Metab Pharmacokinet. 2016. PMID: 25669445
-
Improved dissolution of oleanolic acid with ternary solid dispersions.AAPS PharmSciTech. 2007 Dec 21;8(4):E113. doi: 10.1208/pt0804113. AAPS PharmSciTech. 2007. PMID: 18181534 Free PMC article.
-
Advancing Drug Delivery Paradigms: Polyvinyl Pyrolidone (PVP)-Based Amorphous Solid Dispersion for Enhanced Physicochemical Properties and Therapeutic Efficacy.Polymers (Basel). 2024 Jan 20;16(2):286. doi: 10.3390/polym16020286. Polymers (Basel). 2024. PMID: 38276694 Free PMC article. Review.
-
Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems.Pharmaceutics. 2024 May 21;16(6):692. doi: 10.3390/pharmaceutics16060692. Pharmaceutics. 2024. PMID: 38931816 Free PMC article. Review.
Cited by
-
Effervescence Assisted Fusion Technique to Enhance the Solubility of Drugs.AAPS PharmSciTech. 2015 Dec;16(6):1487-94. doi: 10.1208/s12249-015-0381-2. Epub 2015 Aug 12. AAPS PharmSciTech. 2015. PMID: 26265190 Free PMC article.
-
A New Ligustrazine Derivative-Selective Cytotoxicity by Suppression of NF-κB/p65 and COX-2 Expression on Human Hepatoma Cells. Part 3.Int J Mol Sci. 2015 Jul 17;16(7):16401-13. doi: 10.3390/ijms160716401. Int J Mol Sci. 2015. PMID: 26193270 Free PMC article.
-
Amino acid derivatives of ligustrazine-oleanolic acid as new cytotoxic agents.Molecules. 2014 Nov 7;19(11):18215-31. doi: 10.3390/molecules191118215. Molecules. 2014. PMID: 25387350 Free PMC article.
-
A Series of New Ligustrazine-Triterpenes Derivatives as Anti-Tumor Agents: Design, Synthesis, and Biological Evaluation.Int J Mol Sci. 2015 Sep 2;16(9):21035-55. doi: 10.3390/ijms160921035. Int J Mol Sci. 2015. PMID: 26404253 Free PMC article.
-
Solubility and Permeability Enhancement of Oleanolic Acid by Solid Dispersion in Poloxamers and γ-CD.Molecules. 2022 May 9;27(9):3042. doi: 10.3390/molecules27093042. Molecules. 2022. PMID: 35566392 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

