Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination
- PMID: 23637158
- PMCID: PMC3697481
- DOI: 10.1523/JNEUROSCI.4561-12.2013
Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination
Abstract
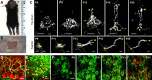
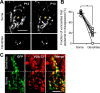
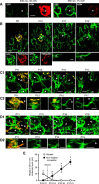
In many regions of the developing mammalian nervous system, functional synaptic circuitry is formed by competitive elimination of early formed redundant synapses. However, how winning synapses emerge through competition remains unclear in the brain largely because of the technical difficulty of directly observing this dynamic cellular process in vivo. Here, we developed a method of two-photon multicolor vital imaging to observe competitive elimination of supernumerary climbing fibers (CFs) in the cerebellum of live mouse pups. At birth, each Purkinje cell (PC) in the cerebellar cortex is innervated by multiple CFs; an activity-dependent regression of supernumerary CFs ultimately yields a single innervation for most PCs by postnatal day 21. As supernumerary CFs are pruned, the terminal field of CFs translocates from the soma to the dendrites of PCs. In vivo time-lapse imaging of CF elimination revealed that (1) CF terminals were highly motile on the soma, but their motility was significantly reduced on dendrites; (2) only one CF could translocate to the dendrites whereas their competitors were restricted to perisomatic regions; and (3) the CF that began dendritic translocation became the winner. Moreover, selective photo-ablation of the winning CF (that undergoes dendritic translocation) reversed the fate of its losing competitor. These results indicate that dendritic translocation is a key cellular event that determines the winner during CF elimination. We propose that CF terminals are selectively stabilized on dendrites, providing irreversible competitive vigor to the first CF to form dendritic synapses.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
