Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice
- PMID: 23637159
- PMCID: PMC6618962
- DOI: 10.1523/JNEUROSCI.0091-13.2013
Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice
Abstract
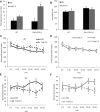
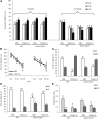
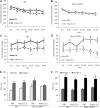
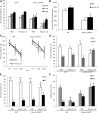
Schizophrenia is thought to result from interactions between susceptible genotypes and environmental risk factors. DISC1 is an important gene for schizophrenia and mood disorders based on both human and animal studies. In the present study we sought to investigate interactions between two distinct point mutations in the mouse Disc1 gene (L100P and Q31L) and maternal immune activation (MIA) during pregnancy with polyinosinic:polycytidylic acid (polyI:C). PolyI:C given at 5 mg/kg impaired cognitive and social behavior in both wild-type (WT) and Disc1-Q31L(+/-) offspring, and reduced prepulse inhibition at 16 but not 8 weeks of age. Disc1-L100P(+/-) mutants were more sensitive to MIA than WT or Disc1-Q31L(+/-) mice. Interleukin-6 (IL-6) is a critical cytokine for mediating the behavioral and transcriptional effects of polyI:C. We found a more pronounced increase of IL-6 in response to polyI:C in fetal brain in Disc1-L100P(+/-) mice compared with WT or Disc1-Q31L(+/-) mice. Coadministration of an anti-IL-6 antibody with polyI:C reversed schizophrenia-related behavioral phenotypes in Disc1-L100P(+/-) mice. In summary, we found specific interactions between discrete genetic (Disc1-L100P(+/-)) and environmental factors (MIA) that exacerbate schizophrenia-related phenotypes. IL-6 may be important in the pathophysiology of this interaction.
Figures
References
-
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
