The Cav3-Kv4 complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations
- PMID: 23637173
- PMCID: PMC6618953
- DOI: 10.1523/JNEUROSCI.5384-12.2013
The Cav3-Kv4 complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations
Abstract
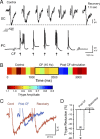
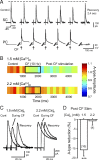
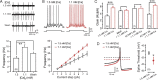
Synaptic transmission and neuronal excitability depend on the concentration of extracellular calcium ([Ca](o)), yet repetitive synaptic input is known to decrease [Ca](o) in numerous brain regions. In the cerebellar molecular layer, synaptic input reduces [Ca](o) by up to 0.4 mm in the vicinity of stellate cell interneurons and Purkinje cell dendrites. The mechanisms used to maintain network excitability and Purkinje cell output in the face of this rapid change in calcium gradient have remained an enigma. Here we use single and dual patch recordings in an in vitro slice preparation of Sprague Dawley rats to investigate the effects of physiological decreases in [Ca](o) on the excitability of cerebellar stellate cells and their inhibitory regulation of Purkinje cells. We find that a Ca(v)3-K(v)4 ion channel complex expressed in stellate cells acts as a calcium sensor that responds to a decrease in [Ca]o by dynamically adjusting stellate cell output to maintain inhibitory charge transfer to Purkinje cells. The Ca(v)3-K(v)4 complex thus enables an adaptive regulation of inhibitory input to Purkinje cells during fluctuations in [Ca](o), providing a homeostatic control mechanism to regulate Purkinje cell excitability during repetitive afferent activity.
Figures






References
-
- Anderson D, Rehak R, Hameed S, Mehaffey WH, Zamponi GW, Turner RW. Regulation of the Kv4.2 complex by CaV3.1 calcium channels. Channels (Austin) 2010a;4:163–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
