Hypofunction of glutamatergic neurotransmission in the periaqueductal gray contributes to nerve-injury-induced neuropathic pain
- PMID: 23637174
- PMCID: PMC6618956
- DOI: 10.1523/JNEUROSCI.5583-12.2013
Hypofunction of glutamatergic neurotransmission in the periaqueductal gray contributes to nerve-injury-induced neuropathic pain
Abstract
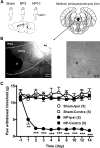
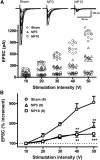
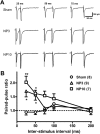
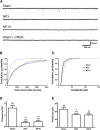
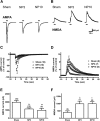
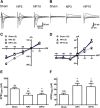
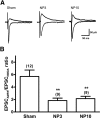
Neuropathic pain, a chronic pain due to neuronal lesion, remains unaltered even after the injury-induced spinal afferent discharges have declined, suggesting an involvement of supraspinal dysfunction. The midbrain ventrolateral periaqueductal gray (vlPAG) is known to be a crucial supraspinal region for initiating descending pain inhibition, but its role in neuropathic pain remains unclear. Therefore, here we examined neuroplastic changes in the vlPAG of midbrain slices isolated from neuropathic rats induced by L5/L6 spinal nerve ligation (SNL) via electrophysiological and neurochemical approaches. Significant mechanical hypersensitivity was induced in rats 2 d after SNL and lasted for >14 d. Compared with the sham-operated group, vlPAG slices from neuropathic rats 3 and 10 days after SNL displayed smaller EPSCs with prolonged latency, less frequent and smaller miniature EPSCs, higher paired-pulse ratio of EPSCs, smaller AMPAR-mediated EPSCs, smaller AMPA currents, greater NMDAR-mediated EPSCs, greater NMDA currents, lower AMPAR-mediated/NMDAR-mediated ratios, and upregulation of the NR1 and NR2B subunits, but not the NR2A, GluR1, or GluR2 subunits, of glutamate receptors. There were no significant differences between day 3 and day 10 neuropathic groups. These results suggest that SNL leads to hypoglutamatergic neurotransmission in the vlPAG resulting from both presynaptic and postsynaptic mechanisms. Upregulation of NMDARs might contribute to hypofunction of AMPARs via subcellular redistribution. Long-term hypoglutamatergic function in the vlPAG may lead to persistent reduction of descending pain inhibition, resulting in chronic neuropathic pain.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
