The role of MuSK in synapse formation and neuromuscular disease
- PMID: 23637281
- PMCID: PMC3632064
- DOI: 10.1101/cshperspect.a009167
The role of MuSK in synapse formation and neuromuscular disease
Abstract
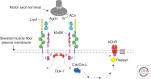
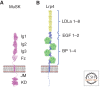
Muscle-specific kinase (MuSK) is essential for each step in neuromuscular synapse formation. Before innervation, MuSK initiates postsynaptic differentiation, priming the muscle for synapse formation. Approaching motor axons recognize the primed, or prepatterned, region of muscle, causing motor axons to stop growing and differentiate into specialized nerve terminals. MuSK controls presynaptic differentiation by causing the clustering of Lrp4, which functions as a direct retrograde signal for presynaptic differentiation. Developing synapses are stabilized by neuronal Agrin, which is released by motor nerve terminals and binds to Lrp4, a member of the low-density lipoprotein receptor family, stimulating further association between Lrp4 and MuSK and increasing MuSK kinase activity. In addition, MuSK phosphorylation is stimulated by an inside-out ligand, docking protein-7 (Dok-7), which is recruited to tyrosine-phosphorylated MuSK and increases MuSK kinase activity. Mutations in MuSK and in genes that function in the MuSK signaling pathway, including Dok-7, cause congenital myasthenia, and autoantibodies to MuSK, Lrp4, and acetylcholine receptors are responsible for myasthenia gravis.
Figures



References
-
- Arber S, Burden SJ, Harris AJ 2002. Patterning of skeletal muscle. Curr Opin Neurobiol 12: 100–103 - PubMed
-
- Beeson D, Higuchi O, Palace J, Cossins J, Spearman H, Maxwell S, Newsom-Davis J, Burke G, Fawcett P, Motomura M, et al. 2006. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 313: 1975–1978 - PubMed
-
- Burden SJ 1993. Synapse-specific gene expression. Trends Genet 9: 12–16 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
