Development of an internally controlled real-time reverse transcriptase PCR assay for pan-dengue virus detection and comparison of four molecular dengue virus detection assays
- PMID: 23637298
- PMCID: PMC3697727
- DOI: 10.1128/JCM.00548-13
Development of an internally controlled real-time reverse transcriptase PCR assay for pan-dengue virus detection and comparison of four molecular dengue virus detection assays
Abstract
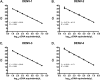
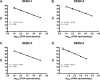
A number of diagnostic tests are available for dengue virus (DENV) detection, including a variety of nucleic acid amplification tests (NAATs). However, reports describing a direct comparison of different NAATs have been limited. In this study, we report the design of an internally controlled real-time reverse transcriptase PCR (rRT-PCR) that detects all four DENV serotypes but does not distinguish between them (the pan-DENV assay). Two hundred clinical samples were then tested using four different DENV RT-PCR assays: the pan-DENV assay, a commercially produced, internally controlled DENV rRT-PCR (the Altona assay), a widely used heminested RT-PCR, and a serotype-specific multiplex rRT-PCR assay. The pan-DENV assay had a linear range extending from 1.0 to 7.0 log10 cDNA equivalents/μl and a lower limit of 95% detection ranging from 1.7 to 7.6 cDNA equivalents/μl, depending on the serotype. When measured against a composite reference standard, the pan-DENV assay proved to be more clinically sensitive than either the Altona or heminested assays, with a sensitivity of 98.0% compared to 72.3% and 78.8%, respectively (P ≤ 0.0001 for both comparisons). The pan-DENV assay detected DENV in significantly more samples collected on or after day 5 of illness and in a subgroup of patients with detectable anti-DENV IgM at presentation. No significant difference in sensitivity was observed between the pan-DENV assay and the multiplex rRT-PCR, despite the presence of an internal control in the former. The detection of DENV RNA late in the course of clinical illness should serve to lengthen the period during which a confirmed molecular diagnosis of DENV infection can be provided.
Figures
References
-
- World Health Organization 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. WHO Press, Geneva, Switzerland - PubMed
-
- World Health Organization 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed World Health Organization, Geneva, Switzerland
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

