Development and evaluation of a next-generation digital PCR diagnostic assay for ocular Chlamydia trachomatis infections
- PMID: 23637300
- PMCID: PMC3697714
- DOI: 10.1128/JCM.00622-13
Development and evaluation of a next-generation digital PCR diagnostic assay for ocular Chlamydia trachomatis infections
Abstract
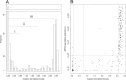
Droplet digital PCR (ddPCR) is an emulsion PCR process that performs absolute quantitation of nucleic acids. We developed a ddPCR assay for Chlamydia trachomatis infections and found it to be accurate and precise. Using PCR mixtures containing plasmids engineered to include the PCR target sequences, we were able to quantify with a dynamic range between 0.07 and 3,160 targets/μl (r(2) = 0.9927) with >95% confidence. Using 1,509 clinical conjunctival swab samples from a population in which trachoma is endemic in Guinea Bissau, we evaluated the specificity and sensitivity of the quantitative ddPCR assay in diagnosing ocular C. trachomatis infections by comparing the performances of ddPCR and the Roche Amplicor CT/NG test. We defined ddPCR tests as positive when we had ≥95% confidence in a nonzero estimate of target load. The sensitivity of ddPCR against Amplicor was 73.3% (95% confidence interval [CI], 67.9 to 78.7%), and specificity was 99.1% (95% CI, 98.6 to 99.6%). Negative and positive predictive values were 94.6% (95% CI, 93.4 to 95.8%) and 94.5% (95% CI, 91.3 to 97.7%), respectively. Based on Amplicor CT/NG testing, the estimated population prevalence of C. trachomatis ocular infection was ∼17.5%. Receiver-operator curve analysis was used to select critical cutoff values for use in clinical settings in which a balance between higher sensitivity and specificity is required. We concluded that ddPCR is an effective diagnostic technology suitable for both research and clinical use in diagnosing ocular C. trachomatis infections.
Figures


Comment in
-
Will droplet digital PCR become the test of choice for detecting and quantifying ocular Chlamydia trachomatis infection? Maybe not.Expert Rev Mol Diagn. 2013 Nov;13(8):789-92. doi: 10.1586/14737159.2013.847792. Epub 2013 Oct 17. Expert Rev Mol Diagn. 2013. PMID: 24134626
References
-
- WHO 2012. Global WHO alliance for the elimination of blinding trachoma by 2020. Wkly. Epidemiol. Rec. 87:161–168 - PubMed
-
- Burton MJ, Holland MJ, Makalo P, Aryee EAN, Alexander NDE, Sillah A, Faal H, West SK, Foster A, Johnson GJ, Mabey DCW, Bailey RL. 2005. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet 365:1321–1328 - PubMed
-
- Chidambaram JD, Alemayehu W, Melese M, Lakew T, Yi E, House J, Cevallos V, Zhou Z, Maxey K, Lee DC, Shapiro BL, Srinivasan M, Porco T, Whitcher JP, Gaynor BD, Lietman TM. 2006. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA 295:1142–1146 - PubMed
-
- Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, Vitale S, Quinn TC, Sheta A, Sallam S, Mkocha H, Faal H. 1999. Azithromycin in control of trachoma. Lancet 354:630–635 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

