Induction of an antiviral state and attenuated coxsackievirus replication in type III interferon-treated primary human pancreatic islets
- PMID: 23637411
- PMCID: PMC3700265
- DOI: 10.1128/JVI.03431-12
Induction of an antiviral state and attenuated coxsackievirus replication in type III interferon-treated primary human pancreatic islets
Abstract
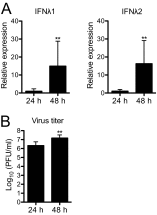
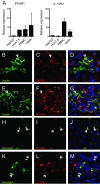
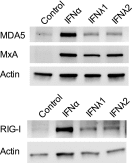
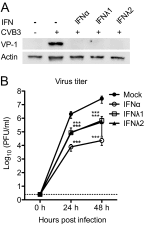
Type III interferons (IFNs), also called lambda interferons (IFN-λ), comprise three isoforms, IFN-λ1 (interleukin-29 [IL-29]), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B). Only limited information is available on their expression and biological functions in humans. Type I and type II IFNs protect human pancreatic islets against coxsackievirus infection, and this is important since such viruses have been proposed to play a role in the development of human type 1 diabetes. Here we investigated whether type III IFN is expressed during infection of human islet cells with coxsackievirus and if type III IFN regulates permissiveness to such infections. We show that human islets respond to a coxsackievirus serotype B3 (CVB3) infection by inducing the expression of type III IFNs. We also demonstrate that islet endocrine cells from nondiabetic individuals express the type III IFN receptor subunits IFN-λR1 and IL-10R2. Pancreatic alpha cells express both receptor subunits, while pancreatic beta cells express only IL-10R2. Type III IFN stimulation elicited a biological response in human islets as indicated by the upregulated expression of antiviral genes as well as pattern recognition receptors. We also show that type III IFN significantly reduces CVB3 replication. Our studies reveal that type III IFNs are expressed during CVB3 infection and that the expression of the type III IFN receptor by the human pancreatic islet allows this group of IFNs to regulate the islets' permissiveness to infection. Our novel observations suggest that type III IFNs may regulate viral replication and thereby contribute to reduced tissue damage and promote islet cell survival during coxsackievirus infection.
Figures
Similar articles
-
Type III interferons are expressed by Coxsackievirus-infected human primary hepatocytes and regulate hepatocyte permissiveness to infection.Clin Exp Immunol. 2014 Sep;177(3):687-95. doi: 10.1111/cei.12368. Clin Exp Immunol. 2014. PMID: 24773058 Free PMC article.
-
Interferons induce an antiviral state in human pancreatic islet cells.Virology. 2007 Oct 10;367(1):92-101. doi: 10.1016/j.virol.2007.05.010. Epub 2007 Jun 8. Virology. 2007. PMID: 17559902
-
Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells.J Virol. 2000 Nov;74(21):10153-64. doi: 10.1128/jvi.74.21.10153-10164.2000. J Virol. 2000. PMID: 11024144 Free PMC article.
-
Novel type III interferons produce anti-tumor effects through multiple functions.Front Biosci (Landmark Ed). 2013 Jun 1;18(3):909-18. doi: 10.2741/4152. Front Biosci (Landmark Ed). 2013. PMID: 23747856 Review.
-
Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses.J Leukoc Biol. 2009 Jul;86(1):23-32. doi: 10.1189/jlb.1208761. Epub 2009 Apr 30. J Leukoc Biol. 2009. PMID: 19304895 Review.
Cited by
-
Coxsackievirus B Persistence Modifies the Proteome and the Secretome of Pancreatic Ductal Cells.iScience. 2019 Sep 27;19:340-357. doi: 10.1016/j.isci.2019.07.040. Epub 2019 Jul 29. iScience. 2019. PMID: 31404834 Free PMC article.
-
Temporal regulation of interferon signalling in human EndoC-βH1 cells.J Mol Endocrinol. 2022 May 19;69(2):299-313. doi: 10.1530/JME-21-0224. J Mol Endocrinol. 2022. PMID: 35521765 Free PMC article.
-
Enteroviral proteases: structure, host interactions and pathogenicity.Rev Med Virol. 2016 Jul;26(4):251-67. doi: 10.1002/rmv.1883. Epub 2016 May 4. Rev Med Virol. 2016. PMID: 27145174 Free PMC article. Review.
-
The innate immune receptor MDA5 limits rotavirus infection but promotes cell death and pancreatic inflammation.EMBO J. 2017 Sep 15;36(18):2742-2757. doi: 10.15252/embj.201696273. Epub 2017 Aug 29. EMBO J. 2017. PMID: 28851763 Free PMC article.
-
An IFIH1 gene polymorphism associated with risk for autoimmunity regulates canonical antiviral defence pathways in Coxsackievirus infected human pancreatic islets.Sci Rep. 2016 Dec 21;6:39378. doi: 10.1038/srep39378. Sci Rep. 2016. PMID: 28000722 Free PMC article.
References
-
- Isaacs A, Lindenmann J. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258–267 - PubMed
-
- Stetson DB, Medzhitov R. 2006. Type I interferons in host defense. Immunity 25:373–381 - PubMed
-
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69–77 - PubMed
-
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

