Metabolomics reveals that tumor xenografts induce liver dysfunction
- PMID: 23637421
- PMCID: PMC3734574
- DOI: 10.1074/mcp.M113.028324
Metabolomics reveals that tumor xenografts induce liver dysfunction
Abstract
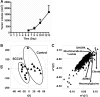
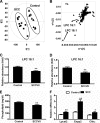
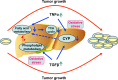
Metabolomics, based on ultraperformance liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry, was used to explore metabolic signatures of tumor growth in mice. Urine samples were collected from control mice and mice injected with squamous cell carcinoma (SCCVII) tumor cells. When tumors reached ∼2 cm, all mice were killed and blood and liver samples collected. The urine metabolites hexanoylglycine, nicotinamide 1-oxide, and 11β,20α-dihydroxy-3-oxopregn-4-en-21-oic acid were elevated in tumor-bearing mice, as was asymmetric dimethylarginine, a biomarker for oxidative stress. Interestingly, SCCVII tumor growth resulted in hepatomegaly, reduced albumin/globulin ratios, and elevated serum triglycerides, suggesting liver dysfunction. Alterations in liver metabolites between SCCVII-tumor-bearing and control mice confirmed the presence of liver injury. Hepatic mRNA analysis indicated that inflammatory cytokines, tumor necrosis factor α, and transforming growth factor β were enhanced in SCCVII-tumor-bearing mice, and the expression of cytochromes P450 was decreased in tumor-bearing mice. Further, genes involved in fatty acid oxidation were decreased, suggesting impaired fatty acid oxidation in SCCVII-tumor-bearing mice. Additionally, activated phospholipid metabolism and a disrupted tricarboxylic acid cycle were observed in SCCVII-tumor-bearing mice. These data suggest that tumor growth imposes a global inflammatory response that results in liver dysfunction and underscore the use of metabolomics to temporally examine these changes and potentially use metabolite changes to monitor tumor treatment response.
Figures



References
-
- Chen C., Krausz K. W., Shah Y. M., Idle J. R., Gonzalez F. J. (2009) Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem. Res. Toxicol. 22, 699–707 - PMC - PubMed
-
- Patterson A. D., Bonzo J. A., Li F., Krausz K. W., Eichler G. S., Aslam S., Tigno X., Weinstein J. N., Hansen B. C., Idle J. R., Gonzalez F. J. (2011) Metabolomics reveals attenuation of the SLC6A20 kidney transporter in nonhuman primate and mouse models of type 2 diabetes mellitus. J. Biol. Chem. 286, 19511–19522 - PMC - PubMed
-
- Monteiro M. S., Carvalho M., Bastos M. D., de Pinho P. G. (2013) Metabolomics analysis for biomarker discovery: advances and challenges. Curr. Med. Chem. 19, 5601–5606 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

