Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling
- PMID: 23637464
- PMCID: PMC3681703
- DOI: 10.1091/mbc.E13-03-0121
Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling
Abstract
The mitochondrial-specific lipid cardiolipin (CL) is required for numerous processes therein. After its synthesis on the matrix-facing leaflet of the inner membrane (IM), CL undergoes acyl chain remodeling to achieve its final form. In yeast, this process is completed by the transacylase tafazzin, which associates with intermembrane space (IMS)-facing membrane leaflets. Mutations in TAZ1 result in the X-linked cardiomyopathy Barth syndrome. Amazingly, despite this clear pathophysiological association, the physiological importance of CL remodeling is unresolved. In this paper, we show that the lipase initiating CL remodeling, Cld1p, is associated with the matrix-facing leaflet of the mitochondrial IM. Thus monolysocardiolipin generated by Cld1p must be transported to IMS-facing membrane leaflets to gain access to tafazzin, identifying a previously unknown step required for CL remodeling. Additionally, we show that Cld1p is the major site of regulation in CL remodeling; and that, like CL biosynthesis, CL remodeling is augmented in growth conditions requiring mitochondrially produced energy. However, unlike CL biosynthesis, dissipation of the mitochondrial membrane potential stimulates CL remodeling, identifying a novel feedback mechanism linking CL remodeling to oxidative phosphorylation capacity.
Figures



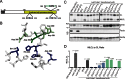

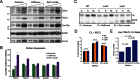
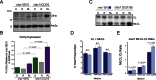
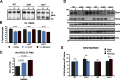
References
-
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

