Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials
- PMID: 23637579
- PMCID: PMC3640084
- DOI: 10.1371/journal.pmed.1001436
Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials
Abstract
Background: Calcimimetic agents lower serum parathyroid hormone levels in people with chronic kidney disease (CKD), but treatment effects on patient-relevant outcomes are uncertain. We conducted a systematic review and meta-analysis to summarize the benefits and harms of calcimimetic therapy in adults with CKD and used cumulative meta-analysis to identify how evidence for calcimimetic treatment has developed in this clinical setting.
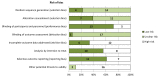
Methods and findings: Cochrane and Embase databases (through February 7, 2013) were electronically searched to identify randomized trials evaluating effects of calcimimetic therapy on mortality and adverse events in adults with CKD. Two independent reviewers identified trials, extracted data, and assessed risk of bias. Eighteen trials comprising 7,446 participants compared cinacalcet plus conventional therapy with placebo or no treatment plus conventional therapy in adults with CKD. In moderate- to high-quality evidence (based on Grading of Recommendations Assessment, Development, and Evaluation criteria) in adults with CKD stage 5D (dialysis), cinacalcet had little or no effect on all-cause mortality (relative risk, 0.97 [95% confidence interval, 0.89-1.05]), had imprecise effect on cardiovascular mortality (0.67 [0.16-2.87]), and prevented parathyroidectomy (0.49 [0.40-0.59]) and hypercalcemia (0.23 [0.05-0.97]), but increased hypocalcemia (6.98 [5.10-9.53]), nausea (2.02 [1.45-2.81]), and vomiting (1.97 [1.73-2.24]). Data for clinical outcomes were sparse in adults with CKD stages 3-5. On average, treating 1,000 people with CKD stage 5D for 1 y had no effect on survival and prevented about three patients from experiencing parathyroidectomy, whilst 60 experienced hypocalcemia and 150 experienced nausea. Analyses were limited by insufficient data in CKD stages 3-5 and kidney transplant recipients.
Conclusions: Cinacalcet reduces the need for parathyroidectomy in patients with CKD stage 5D, but does not appear to improve all-cause or cardiovascular mortality. Additional trials in CKD stage 5D are unlikely to change our confidence in the treatment effects of cinacalcet in this population.
Conflict of interest statement
MT holds research grants from Amgen, which manufactures cinacalcet. AC received speaker honoraria from Amgen and Abbott, and is a member of the European Renal Best Practices Board. The remaining authors declare that no competing interests exist.
Figures

Comment in
-
Dialysis: Effect of cinacalcet on survival--the saga continues.Nat Rev Nephrol. 2013 Aug;9(8):435-6. doi: 10.1038/nrneph.2013.130. Epub 2013 Jul 2. Nat Rev Nephrol. 2013. PMID: 23820810 No abstract available.
References
-
- National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39 (2 Suppl 1): S1–S266. - PubMed
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305. - PubMed
-
- Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, et al. (2010) Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429. - PubMed
-
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases (2012) 2012 USRDS annual data report: atlas of chronic kidney disease in the United States. Bethesda (Maryland): National Institutes of Health.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

