Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus
- PMID: 23637602
- PMCID: PMC3630103
- DOI: 10.1371/journal.ppat.1003312
Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus
Abstract
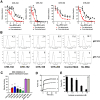
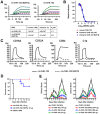
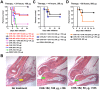
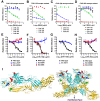
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that causes global epidemics of a debilitating polyarthritis in humans. As there is a pressing need for the development of therapeutic agents, we screened 230 new mouse anti-CHIKV monoclonal antibodies (MAbs) for their ability to inhibit infection of all three CHIKV genotypes. Four of 36 neutralizing MAbs (CHK-102, CHK-152, CHK-166, and CHK-263) provided complete protection against lethality as prophylaxis in highly susceptible immunocompromised mice lacking the type I IFN receptor (Ifnar(-/-) ) and mapped to distinct epitopes on the E1 and E2 structural proteins. CHK-152, the most protective MAb, was humanized, shown to block viral fusion, and require Fc effector function for optimal activity in vivo. In post-exposure therapeutic trials, administration of a single dose of a combination of two neutralizing MAbs (CHK-102+CHK-152 or CHK-166+CHK-152) limited the development of resistance and protected immunocompromised mice against disease when given 24 to 36 hours before CHIKV-induced death. Selected pairs of highly neutralizing MAbs may be a promising treatment option for CHIKV in humans.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: S. Johnson and S. Gorlatov are employees of MacroGenics, which has an option for licensure of the anti-CHKV MAbs for commercial development. M. Diamond is a paid consultant for MacroGenics. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures


References
-
- Staples JE, Breiman RF, Powers AM (2009) Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis 49: 942–948. - PubMed
-
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT (2012) Chikungunya: a re-emerging virus. Lancet 379: 662–671. - PubMed
-
- Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, et al. (2007) Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J Gen Virol 88: 1967–1976. - PubMed
-
- Powers AM, Brault AC, Tesh RB, Weaver SC (2000) Re-emergence of Chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol 81: 471–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

