Sensory neuron-derived eph regulates glomerular arbors and modulatory function of a central serotonergic neuron
- PMID: 23637622
- PMCID: PMC3630106
- DOI: 10.1371/journal.pgen.1003452
Sensory neuron-derived eph regulates glomerular arbors and modulatory function of a central serotonergic neuron
Erratum in
-
Correction: Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron.PLoS Genet. 2017 Nov 1;13(11):e1007083. doi: 10.1371/journal.pgen.1007083. eCollection 2017 Nov. PLoS Genet. 2017. PMID: 29091719 Free PMC article.
Abstract
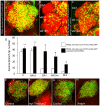
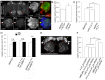
Olfactory sensory neurons connect to the antennal lobe of the fly to create the primary units for processing odor cues, the glomeruli. Unique amongst antennal-lobe neurons is an identified wide-field serotonergic neuron, the contralaterally-projecting, serotonin-immunoreactive deutocerebral neuron (CSDn). The CSDn spreads its termini all over the contralateral antennal lobe, suggesting a diffuse neuromodulatory role. A closer examination, however, reveals a restricted pattern of the CSDn arborization in some glomeruli. We show that sensory neuron-derived Eph interacts with Ephrin in the CSDn, to regulate these arborizations. Behavioural analysis of animals with altered Eph-ephrin signaling and with consequent arborization defects suggests that neuromodulation requires local glomerular-specific patterning of the CSDn termini. Our results show the importance of developmental regulation of terminal arborization of even the diffuse modulatory neurons to allow them to route sensory-inputs according to the behavioural contexts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Identified Serotonergic Modulatory Neurons Have Heterogeneous Synaptic Connectivity within the Olfactory System of Drosophila.J Neurosci. 2017 Aug 2;37(31):7318-7331. doi: 10.1523/JNEUROSCI.0192-17.2017. Epub 2017 Jun 28. J Neurosci. 2017. PMID: 28659283 Free PMC article.
-
The Wiring Logic of an Identified Serotonergic Neuron That Spans Sensory Networks.J Neurosci. 2020 Aug 12;40(33):6309-6327. doi: 10.1523/JNEUROSCI.0552-20.2020. Epub 2020 Jul 8. J Neurosci. 2020. PMID: 32641403 Free PMC article.
-
Local synaptic inputs support opposing, network-specific odor representations in a widely projecting modulatory neuron.Elife. 2019 Jul 2;8:e46839. doi: 10.7554/eLife.46839. Elife. 2019. PMID: 31264962 Free PMC article.
-
Olfactory maps and odor images.Curr Opin Neurobiol. 2002 Aug;12(4):387-92. doi: 10.1016/s0959-4388(02)00348-3. Curr Opin Neurobiol. 2002. PMID: 12139985 Review.
-
Central olfactory pathways in mosquitoes and other insects.Ciba Found Symp. 1996;200:184-92; discussion 192-6, 226-32. doi: 10.1002/9780470514948.ch14. Ciba Found Symp. 1996. PMID: 8894298 Review.
Cited by
-
Development of connectivity in a motoneuronal network in Drosophila larvae.Curr Biol. 2015 Mar 2;25(5):568-76. doi: 10.1016/j.cub.2014.12.056. Epub 2015 Feb 19. Curr Biol. 2015. PMID: 25702582 Free PMC article.
-
A Single Pair of Serotonergic Neurons Counteracts Serotonergic Inhibition of Ethanol Attraction in Drosophila.PLoS One. 2016 Dec 9;11(12):e0167518. doi: 10.1371/journal.pone.0167518. eCollection 2016. PLoS One. 2016. PMID: 27936023 Free PMC article.
-
Functional integration of a serotonergic neuron in the Drosophila antennal lobe.Elife. 2016 Aug 30;5:e16836. doi: 10.7554/eLife.16836. Elife. 2016. PMID: 27572257 Free PMC article.
-
Dendritic Eph organizes dendrodendritic segregation in discrete olfactory map formation in Drosophila.Genes Dev. 2017 May 15;31(10):1054-1065. doi: 10.1101/gad.297424.117. Genes Dev. 2017. PMID: 28637694 Free PMC article.
-
The anatomical basis for modulatory convergence in the antennal lobe of Manduca sexta.J Comp Neurol. 2016 Jun 15;524(9):1859-75. doi: 10.1002/cne.23926. Epub 2015 Dec 29. J Comp Neurol. 2016. PMID: 26560074 Free PMC article.
References
-
- Azmitia EC (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain research bulletin 56: 413–424. - PubMed
-
- Jacobs BL, Azmitia EC (1992) Structure and function of the brain serotonin system. Physiological reviews 72: 165–229. - PubMed
-
- Lucki I (1998) The spectrum of behaviors influenced by serotonin. Biological psychiatry 44: 151–162. - PubMed
-
- Jacobs BL, Fornal CA (1997) Serotonin and motor activity. Current opinion in neurobiology 7: 820–825. - PubMed
-
- Jacobs BL, Fornal CA (1999) Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 21: 9S–15S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

