Scavenger receptors mediate the role of SUMO and Ftz-f1 in Drosophila steroidogenesis
- PMID: 23637637
- PMCID: PMC3630131
- DOI: 10.1371/journal.pgen.1003473
Scavenger receptors mediate the role of SUMO and Ftz-f1 in Drosophila steroidogenesis
Abstract
SUMOylation participates in ecdysteroid biosynthesis at the onset of metamorphosis in Drosophila melanogaster. Silencing the Drosophila SUMO homologue smt3 in the prothoracic gland leads to reduced lipid content, low ecdysone titers, and a block in the larval-pupal transition. Here we show that the SR-BI family of Scavenger Receptors mediates SUMO functions. Reduced levels of Snmp1 compromise lipid uptake in the prothoracic gland. In addition, overexpression of Snmp1 is able to recover lipid droplet levels in the smt3 knockdown prothoracic gland cells. Snmp1 expression depends on Ftz-f1 (an NR5A-type orphan nuclear receptor), the expression of which, in turn, depends on SUMO. Furthermore, we show by in vitro and in vivo experiments that Ftz-f1 is SUMOylated. RNAi-mediated knockdown of ftz-f1 phenocopies that of smt3 at the larval to pupal transition, thus Ftz-f1 is an interesting candidate to mediate some of the functions of SUMO at the onset of metamorphosis. Additionally, we demonstrate that the role of SUMOylation, Ftz-f1, and the Scavenger Receptors in lipid capture and mobilization is conserved in other steroidogenic tissues such as the follicle cells of the ovary. smt3 knockdown, as well as ftz-f1 or Scavenger knockdown, depleted the lipid content of the follicle cells, which could be rescued by Snmp1 overexpression. Therefore, our data provide new insights into the regulation of metamorphosis via lipid homeostasis, showing that Drosophila Smt3, Ftz-f1, and SR-BIs are part of a general mechanism for uptake of lipids such as cholesterol, required during development in steroidogenic tissues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


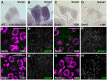





References
-
- Gilbert LI (2004) Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster . Mol Cell Endocrinol 215: 1–10. - PubMed
-
- Rewitz KF, Rybczynski R, Warren JT, LI G (2006) The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans 34: 1256–1260. - PubMed
-
- Thummel CS (2001) Molecular mechanisms of developmental timing in C. elegans and Drosophila . Dev Cell 1: 453–465. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

