A model of electrophysiological heterogeneity in periglomerular cells
- PMID: 23637658
- PMCID: PMC3636461
- DOI: 10.3389/fncom.2013.00049
A model of electrophysiological heterogeneity in periglomerular cells
Abstract
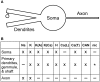
Olfactory bulb (OB) periglomerular (PG) cells are heterogeneous with respect to several features, including morphology, connectivity, patterns of protein expression, and electrophysiological properties. However, these features rarely correlate with one another, suggesting that the differentiating properties of PG cells may arise from multiple independent adaptive variables rather than representing discrete cell classes. We use computational modeling to assess this hypothesis with respect to electrophysiological properties. Specifically, we show that the heterogeneous electrophysiological properties demonstrated in PG cell recordings can be explained solely by differences in the relative expression levels of ion channel species in the cell, without recourse to modifying channel kinetic properties themselves. This PG cell model can therefore be used as the basis for diverse cellular and network-level analyses of OB computations. Moreover, this simple basis for heterogeneity contributes to an emerging hypothesis that glomerular-layer interneurons may be better described as a single population expressing distributions of partially independent, potentially plastic properties, rather than as a set of discrete cell classes.
Keywords: NEURON simulator; acetylcholine; computational model; glomerulus; juxtaglomerular neurons; olfactory bulb.
Figures



References
-
- Cadetti L., Belluzzi O. (2001). Hyperpolarisation-activated current in glomerular cells of the rat olfactory bulb. Neuroreport 12, 3117–3120 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

