Protection of cells against oxidative stress by nanomolar levels of hydroxyflavones indicates a new type of intracellular antioxidant mechanism
- PMID: 23637768
- PMCID: PMC3630532
- DOI: 10.1371/journal.pone.0060796
Protection of cells against oxidative stress by nanomolar levels of hydroxyflavones indicates a new type of intracellular antioxidant mechanism
Abstract
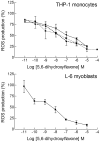
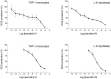
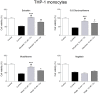
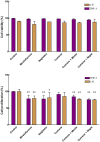
Natural polyphenol compounds are often good antioxidants, but they also cause damage to cells through more or less specific interactions with proteins. To distinguish antioxidant activity from cytotoxic effects we have tested four structurally related hydroxyflavones (baicalein, mosloflavone, negletein, and 5,6-dihydroxyflavone) at very low and physiologically relevant levels, using two different cell lines, L-6 myoblasts and THP-1 monocytes. Measurements using intracellular fluorescent probes and electron paramagnetic resonance spectroscopy in combination with cytotoxicity assays showed strong antioxidant activities for baicalein and 5,6-dihydroxyflavone at picomolar concentrations, while 10 nM partially protected monocytes against the strong oxidative stress induced by 200 µM cumene hydroperoxide. Wide range dose-dependence curves were introduced to characterize and distinguish the mechanism and targets of different flavone antioxidants, and identify cytotoxic effects which only became detectable at micromolar concentrations. Analysis of these dose-dependence curves made it possible to exclude a protein-mediated antioxidant response, as well as a mechanism based on the simple stoichiometric scavenging of radicals. The results demonstrate that these flavones do not act on the same radicals as the flavonol quercetin. Considering the normal concentrations of all the endogenous antioxidants in cells, the addition of picomolar or nanomolar levels of these flavones should not be expected to produce any detectable increase in the total cellular antioxidant capacity. The significant intracellular antioxidant activity observed with 1 pM baicalein means that it must be scavenging radicals that for some reason are not eliminated by the endogenous antioxidants. The strong antioxidant effects found suggest these flavones, as well as quercetin and similar polyphenolic antioxidants, at physiologically relevant concentrations act as redox mediators to enable endogenous antioxidants to reach and scavenge different pools of otherwise inaccessible radicals.
Conflict of interest statement
Figures







References
-
- Rice-Evans CA, Miller NJ (1996) Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans 24: 790–795. - PubMed
-
- Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20: 933–956. - PubMed
-
- Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46: 531–542. - PubMed
-
- Gutteridge JMC, Halliwell B (2010) Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res Commun 393: 561–564. - PubMed
-
- Clifford MN (2004) Diet-derived phenols in plasma and tissues and their implications for health. Planta Med 70: 1103–1114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

