Vertical transmission of respiratory syncytial virus modulates pre- and postnatal innervation and reactivity of rat airways
- PMID: 23637810
- PMCID: PMC3630224
- DOI: 10.1371/journal.pone.0061309
Vertical transmission of respiratory syncytial virus modulates pre- and postnatal innervation and reactivity of rat airways
Abstract
Background: Environmental exposure to respiratory syncytial virus (RSV) is a leading cause of respiratory infections in infants, but it remains unknown whether this infection is transmitted transplacentally from the lungs of infected mothers to the offspring. We sought to test the hypothesis that RSV travels from the respiratory tract during pregnancy, crosses the placenta to the fetus, persists in the lung tissues of the offspring, and modulates pre- and postnatal expression of growth factors, thereby predisposing to airway hyperreactivity.
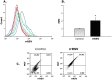
Methodology: Pregnant rats were inoculated intratracheally at midterm using recombinant RSV expressing red fluorescent protein (RFP). Viral RNA was amplified by RT-PCR and confirmed by sequencing. RFP expression was analyzed by flow cytometry and viral culture. Developmental and pathophysiologic implications of prenatal infection were determined by analyzing the expression of genes encoding critical growth factors, particularly neurotrophic factors and receptors. We also measured the expression of key neurotransmitters and postnatal bronchial reactivity in vertically infected lungs, and assessed their dependence on neurotrophic signaling using selective biological or chemical inhibition.
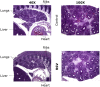
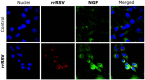
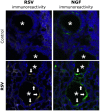
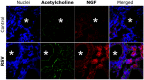
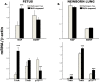
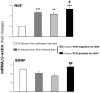
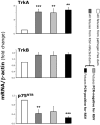
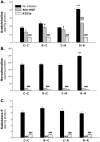
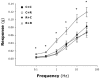
Principal findings: RSV genome was found in 30% of fetuses, as well as in the lungs of 40% of newborns and 25% of adults. RFP expression was also shown by flow cytometry and replicating virus was cultured from exposed fetuses. Nerve growth factor and its TrkA receptor were upregulated in RSV- infected fetal lungs and co-localized with increased cholinergic innervation. Acetylcholine expression and smooth muscle response to cholinergic stimulation increased in lungs exposed to RSV in utero and reinfected after birth, and blocking TrkA signaling inhibited both effects.
Conclusions/significance: Our data show transplacental transmission of RSV from mother to offspring and persistence of vertically transmitted virus in lungs after birth. Exposure to RSV in utero is followed by dysregulation of neurotrophic pathways predisposing to postnatal airway hyperreactivity upon reinfection with the virus.
Conflict of interest statement
Figures


Similar articles
-
Prenatal Exposure to Respiratory Syncytial Virus Alters Postnatal Immunity and Airway Smooth Muscle Contractility during Early-Life Reinfections.PLoS One. 2017 Feb 8;12(2):e0168786. doi: 10.1371/journal.pone.0168786. eCollection 2017. PLoS One. 2017. PMID: 28178290 Free PMC article.
-
Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs?Curr Opin Pharmacol. 2014 Jun;16:82-8. doi: 10.1016/j.coph.2014.03.008. Epub 2014 May 6. Curr Opin Pharmacol. 2014. PMID: 24810284 Free PMC article. Review.
-
Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection.Pediatr Infect Dis J. 2003 Feb;22(2 Suppl):S66-74; discussion S74-5. doi: 10.1097/01.inf.0000053888.67311.1d. Pediatr Infect Dis J. 2003. PMID: 12671455 Review.
-
Persistence of respiratory syncytial virus (RSV) infection and development of RSV-specific IgG1 response in a guinea-pig model of acute bronchiolitis.Eur Respir J. 1997 Jan;10(1):20-6. doi: 10.1183/09031936.97.10010020. Eur Respir J. 1997. PMID: 9032486
-
NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection.PLoS One. 2009 Jul 31;4(7):e6444. doi: 10.1371/journal.pone.0006444. PLoS One. 2009. PMID: 19649262 Free PMC article.
Cited by
-
A 2-year-old girl with chronic crackles after respiratory syncytial virus infection: a case report.J Med Case Rep. 2018 Sep 12;12(1):258. doi: 10.1186/s13256-018-1797-6. J Med Case Rep. 2018. PMID: 30205845 Free PMC article.
-
Potential Neurocognitive Symptoms Due to Respiratory Syncytial Virus Infection.Pathogens. 2021 Dec 31;11(1):47. doi: 10.3390/pathogens11010047. Pathogens. 2021. PMID: 35055995 Free PMC article. Review.
-
Coronavirus entry and release in polarized epithelial cells: a review.Rev Med Virol. 2014 Sep;24(5):308-15. doi: 10.1002/rmv.1792. Epub 2014 Apr 16. Rev Med Virol. 2014. PMID: 24737708 Free PMC article. Review.
-
Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring.Pediatr Res. 2016 Feb;79(2):278-86. doi: 10.1038/pr.2015.226. Epub 2015 Nov 5. Pediatr Res. 2016. PMID: 26539661 Free PMC article.
-
Detection of respiratory syncytial virus (RSV) at birth in a newborn with respiratory distress.Pediatr Pulmonol. 2017 Oct;52(10):E81-E84. doi: 10.1002/ppul.23775. Epub 2017 Aug 18. Pediatr Pulmonol. 2017. PMID: 28834426 Free PMC article.
References
-
- Wright M, Piedimonte G (2011) Respiratory syncytial virus prevention and therapy: Past, present, and future. Pediatr Pulmonol 46: 324–347. - PubMed
-
- Iankevich OD, Dreizin RS, Makhlinovskaia NL, Gorodnitskaia NA (1975) Viremia in respiratory syncytial virus infection. Vopr Virusol 4: 455–458. - PubMed
-
- Liu XM, Wang Z, Guo Y (2007) Respiratory syncytial virus nephropathy in rats. Kidney Int 71: 388–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

