Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21
- PMID: 23637819
- PMCID: PMC3630201
- DOI: 10.1371/journal.pone.0061372
Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21
Abstract
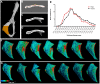
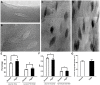
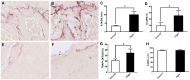
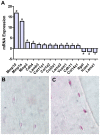
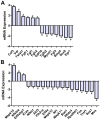
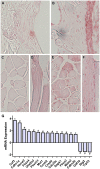
Bone is a dynamically remodeled tissue that requires gravity-mediated mechanical stimulation for maintenance of mineral content and structure. Homeostasis in bone occurs through a balance in the activities and signaling of osteoclasts, osteoblasts, and osteocytes, as well as proliferation and differentiation of their stem cell progenitors. Microgravity and unloading are known to cause osteoclast-mediated bone resorption; however, we hypothesize that osteocytic osteolysis, and cell cycle arrest during osteogenesis may also contribute to bone loss in space. To test this possibility, we exposed 16-week-old female C57BL/6J mice (n = 8) to microgravity for 15-days on the STS-131 space shuttle mission. Analysis of the pelvis by µCT shows decreases in bone volume fraction (BV/TV) of 6.29%, and bone thickness of 11.91%. TRAP-positive osteoclast-covered trabecular bone surfaces also increased in microgravity by 170% (p = 0.004), indicating osteoclastic bone degeneration. High-resolution X-ray nanoCT studies revealed signs of lacunar osteolysis, including increases in cross-sectional area (+17%, p = 0.022), perimeter (+14%, p = 0.008), and canalicular diameter (+6%, p = 0.037). Expression of matrix metalloproteinases (MMP) 1, 3, and 10 in bone, as measured by RT-qPCR, was also up-regulated in microgravity (+12.94, +2.98 and +16.85 fold respectively, p<0.01), with MMP10 localized to osteocytes, and consistent with induction of osteocytic osteolysis. Furthermore, expression of CDKN1a/p21 in bone increased 3.31 fold (p<0.01), and was localized to osteoblasts, possibly inhibiting the cell cycle during tissue regeneration as well as conferring apoptosis resistance to these cells. Finally the apoptosis inducer Trp53 was down-regulated by -1.54 fold (p<0.01), possibly associated with the quiescent survival-promoting function of CDKN1a/p21. In conclusion, our findings identify the pelvic and femoral region of the mouse skeleton as an active site of rapid bone loss in microgravity, and indicate that this loss is not limited to osteoclastic degradation. Therefore, this study offers new evidence for microgravity-induced osteocytic osteolysis, and CDKN1a/p21-mediated osteogenic cell cycle arrest.
Conflict of interest statement
Figures
Similar articles
-
[Osteocytic osteolysis : measurements of the volume of osteocytic lacunae].Clin Calcium. 2012 May;22(5):677-83. Clin Calcium. 2012. PMID: 22549192 Review. Japanese.
-
Evidence that osteocyte perilacunar remodelling contributes to polyethylene wear particle induced osteolysis.Acta Biomater. 2016 Mar;33:242-51. doi: 10.1016/j.actbio.2016.01.016. Epub 2016 Jan 18. Acta Biomater. 2016. PMID: 26796208
-
Evidence for the role of connexin 43-mediated intercellular communication in the process of intracortical bone resorption via osteocytic osteolysis.BMC Musculoskelet Disord. 2014 Apr 9;15:122. doi: 10.1186/1471-2474-15-122. BMC Musculoskelet Disord. 2014. PMID: 24716486 Free PMC article.
-
The lack of mass transfer in bone lacunar-canalicular system may be the decisive factor of osteoporosis under microgravity.Life Sci Space Res (Amst). 2021 Nov;31:80-84. doi: 10.1016/j.lssr.2021.09.002. Epub 2021 Sep 10. Life Sci Space Res (Amst). 2021. PMID: 34689953
-
[Glucocorticoid and Bone. Osteocytic osteolysis : potential modulation by glucocorticoids].Clin Calcium. 2014 Sep;24(9):1337-42. Clin Calcium. 2014. PMID: 25177006 Review. Japanese.
Cited by
-
Bone health in spacefaring rodents and primates: systematic review and meta-analysis.NPJ Microgravity. 2021 Jun 1;7(1):19. doi: 10.1038/s41526-021-00147-7. NPJ Microgravity. 2021. PMID: 34075059 Free PMC article.
-
Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells.FASEB J. 2014 Sep;28(9):4077-87. doi: 10.1096/fj.14-249714. Epub 2014 Jun 5. FASEB J. 2014. PMID: 24903274 Free PMC article.
-
Culture of human cells in experimental units for spaceflight impacts on their behavior.Exp Biol Med (Maywood). 2017 May;242(10):1072-1078. doi: 10.1177/1535370216684039. Epub 2016 Jan 1. Exp Biol Med (Maywood). 2017. PMID: 28492348 Free PMC article.
-
Changes in the intra- and peri-cellular sclerostin distribution in lacuno-canalicular system induced by mechanical unloading.J Bone Miner Metab. 2021 Mar;39(2):148-159. doi: 10.1007/s00774-020-01135-9. Epub 2020 Aug 25. J Bone Miner Metab. 2021. PMID: 32844318
-
MC3T3 infiltration and proliferation in bovine trabecular scaffold regulated by dynamic flow bioreactor and augmented by low-intensity pulsed ultrasound.J Orthop Translat. 2018 Mar 15;14:16-22. doi: 10.1016/j.jot.2018.02.002. eCollection 2018 Jul. J Orthop Translat. 2018. PMID: 30035029 Free PMC article.
References
-
- Nabavi N, Khandani A, Camirand A, Harrison RE (2011) Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 49: 965–974. - PubMed
-
- Hughes-Fulford M (2003) Function of the cytoskeleton in gravisensing during spaceflight. Adv Space Res 32: 1585–1593. - PubMed
-
- Ohshima H (2006) [Bone loss and bone metabolism in astronauts during long-duration space flight]. Clin Calcium 16: 81–85. - PubMed
-
- Smith SM, Heer M (2002) Calcium and bone metabolism during space flight. Nutrition 18: 849–852. - PubMed
-
- Zayzafoon M, Meyers VE, McDonald JM (2005) Microgravity: the immune response and bone. Immunol Rev 208: 267–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

