Gaze holding in healthy subjects
- PMID: 23637824
- PMCID: PMC3637181
- DOI: 10.1371/journal.pone.0061389
Gaze holding in healthy subjects
Abstract
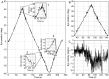
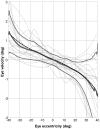
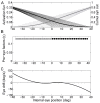
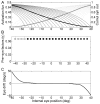
Eccentric gaze in darkness evokes minor centripetal eye drifts in healthy subjects, as cerebellar control sufficiently compensates for the inherent deficiencies of the brainstem gaze-holding network. This behavior is commonly described using a leaky integrator model, which assumes that eye velocity grows linearly with gaze eccentricity. Results from previous studies in patients and healthy subjects suggest caution when this assumption is applied to eye eccentricities larger than 20 degrees. To obtain a detailed characterization of the centripetal gaze-evoked drift, we recorded horizontal eye position in 20 healthy subjects. With their head fixed, they were asked to fixate a flashing dot (50 ms every 2 s)that was quasi-stationary displacing(0.5 deg/s) between ± 40 deg horizontally in otherwise complete darkness. Drift velocity was weak at all angles tested. Linearity was assessed by dividing the range of gaze eccentricity in four bins of 20 deg each, and comparing the slopes of a linear function fitted to the horizontal velocity in each bin. The slopes of single subjects for gaze eccentricities of ± 0-20 deg were, in median,0.41 times the slopes obtained for gaze eccentricities of ± 20-40 deg. By smoothing the individual subjects' eye velocity as a function of gaze eccentricity, we derived a population of position-velocity curves. We show that a tangent function provides a better fit to the mean of these curves when large eccentricities are considered. This implies that the quasi-linear behavior within the typical ocular motor range is the result of a tuning procedure, which is optimized in the most commonly used range of gaze. We hypothesize that the observed non-linearity at eccentric gaze results from a saturation of the input that each neuron in the integrating network receives from the others. As a consequence, gaze-holding performance declines more rapidly at large eccentricities.
Conflict of interest statement
Figures





References
-
- Leigh RJ, Zee DS (2006) The neurology of eye movements. Oxford Press.
-
- Abel LA, Parker L, Daroff RB, Dell'Osso LF (1978a) End-point nystagmus, Invest. Ophthalmol. Vis Sci 17: 539–544. - PubMed
-
- Eizenman M, Cheng P, Sharpe JA, Frecker RC (1990) End-point nystagmus and ocular drift: an experimental and theoretical study. Vision Res 30(6): 863–877. - PubMed
-
- Shallo-Hoffmann J, Schwarze H, Simonsz HJ, Mühlendych H (1990) A reexamination of end-point and rebound nystagmus in normal. Invest Ophthalmol Vis Sci 31(2): 388–392. - PubMed
-
- Whyte CA, Petrock AM, Rosenberg M (2010) Occurrence of physiologic gaze-evoked nystagmus at small angles of gaze. . Invest Ophthalmol Vis Sci 51: 2476–2478. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

