Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model
- PMID: 23637888
- PMCID: PMC3630120
- DOI: 10.1371/journal.pone.0061700
Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model
Abstract
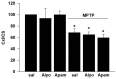
Bee venom has recently been suggested to possess beneficial effects in the treatment of Parkinson disease (PD). For instance, it has been observed that bilateral acupoint stimulation of lower hind limbs with bee venom was protective in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. In particular, a specific component of bee venom, apamin, has previously been shown to have protective effects on dopaminergic neurons in vitro. However, no information regarding a potential protective action of apamin in animal models of PD is available to date. The specific goals of the present study were to (i) establish that the protective effect of bee venom for dopaminergic neurons is not restricted to acupoint stimulation, but can also be observed using a more conventional mode of administration and to (ii) demonstrate that apamin can mimic the protective effects of a bee venom treatment on dopaminergic neurons. Using the chronic mouse model of MPTP/probenecid, we show that bee venom provides sustained protection in an animal model that mimics the chronic degenerative process of PD. Apamin, however, reproduced these protective effects only partially, suggesting that other components of bee venom enhance the protective action of the peptide.
Conflict of interest statement
Figures




Similar articles
-
Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson's disease.Neurol Res. 2010 Feb;32 Suppl 1:88-91. doi: 10.1179/016164109X12537002794282. Neurol Res. 2010. PMID: 20034453
-
Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson's disease: role of regulatory T cells.Brain Behav Immun. 2012 Nov;26(8):1322-30. doi: 10.1016/j.bbi.2012.08.013. Epub 2012 Sep 5. Brain Behav Immun. 2012. PMID: 22974722
-
Comparison of the Protective Effects of Bee Venom Extracts with Varying PLA2 Compositions in a Mouse Model of Parkinson's Disease.Toxins (Basel). 2019 Jun 19;11(6):358. doi: 10.3390/toxins11060358. Toxins (Basel). 2019. PMID: 31248167 Free PMC article.
-
Bee Venom Components as Therapeutic Tools against Prostate Cancer.Toxins (Basel). 2021 May 7;13(5):337. doi: 10.3390/toxins13050337. Toxins (Basel). 2021. PMID: 34067049 Free PMC article. Review.
-
Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease.Toxins (Basel). 2020 Mar 19;12(3):195. doi: 10.3390/toxins12030195. Toxins (Basel). 2020. PMID: 32204567 Free PMC article. Review.
Cited by
-
Clinical Applications of Bee Venom Acupoint Injection.Toxins (Basel). 2020 Sep 27;12(10):618. doi: 10.3390/toxins12100618. Toxins (Basel). 2020. PMID: 32992601 Free PMC article.
-
Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System.Front Mol Neurosci. 2018 Jul 30;11:258. doi: 10.3389/fnmol.2018.00258. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30104956 Free PMC article. Review.
-
Nationwide Survey of Patient Knowledge and Attitudes towards Human Experimentation Using Stem Cells or Bee Venom Acupuncture for Parkinson's Disease.J Mov Disord. 2014 Oct;7(2):84-91. doi: 10.14802/jmd.14012. Epub 2014 Oct 30. J Mov Disord. 2014. PMID: 25360232 Free PMC article.
-
Bee Venom Alleviates Motor Deficits and Modulates the Transfer of Cortical Information through the Basal Ganglia in Rat Models of Parkinson's Disease.PLoS One. 2015 Nov 16;10(11):e0142838. doi: 10.1371/journal.pone.0142838. eCollection 2015. PLoS One. 2015. PMID: 26571268 Free PMC article.
-
Potassium Channels: A Potential Therapeutic Target for Parkinson's Disease.Neurosci Bull. 2018 Apr;34(2):341-348. doi: 10.1007/s12264-017-0177-3. Epub 2017 Sep 7. Neurosci Bull. 2018. PMID: 28884460 Free PMC article. Review.
References
-
- Dauer W, Przedborski S (2003) Parkinson's Disease: Mechanisms and Models. Neuron 39: 889–909. - PubMed
-
- Rascol O, Lozano A, Stern M, Poewe W (2011) Milestones in Parkinson's disease therapeutics. Movement disorders: official journal of the Movement Disorder Society 26: 1072–1082 Available: http://www.ncbi.nlm.nih.gov/pubmed/21626552. Accessed 3 March 2012. - PubMed
-
- Doo A-R, Kim S, Kim S, Moon W, Yin C, et al. (2010) Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1- Parkinson's disease. Neurological Research Suppl 1: 88–91. - PubMed
-
- Kim J, Yang EJ, Lee M, Kim Y, Huh Y, et al. (2011) Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson's disease. Int J Neurosci 121: 209–217. - PubMed
-
- Chen Y-N, Li K-C, Li Z, Shang G-W, Liu DN, et al. (2006) Effects of bee venom peptidergic components on rat pain-related behaviors and inflammation. Neuroscience 138: 631–640 Available: http://www.ncbi.nlm.nih.gov/pubmed/16446039. Accessed 15 June 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

