Toxicological effects of the different substances in tobacco smoke on human embryonic development by a systems chemo-biology approach
- PMID: 23637898
- PMCID: PMC3639264
- DOI: 10.1371/journal.pone.0061743
Toxicological effects of the different substances in tobacco smoke on human embryonic development by a systems chemo-biology approach
Abstract
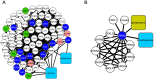
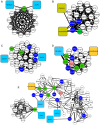
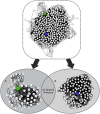
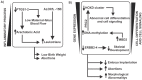
The physiological and molecular effects of tobacco smoke in adult humans and the development of cancer have been well described. In contrast, how tobacco smoke affects embryonic development remains poorly understood. Morphological studies of the fetuses of smoking pregnant women have shown various physical deformities induced by constant fetal exposure to tobacco components, especially nicotine. In addition, nicotine exposure decreases fetal body weight and bone/cartilage growth in addition to decreasing cranial diameter and tibia length. Unfortunately, the molecular pathways leading to these morphological anomalies are not completely understood. In this study, we applied interactome data mining tools and small compound interaction networks to elucidate possible molecular pathways associated with the effects of tobacco smoke components during embryonic development in pregnant female smokers. Our analysis showed a relationship between nicotine and 50 additional harmful substances involved in a variety of biological process that can cause abnormal proliferation, impaired cell differentiation, and increased oxidative stress. We also describe how nicotine can negatively affect retinoic acid signaling and cell differentiation through inhibition of retinoic acid receptors. In addition, nicotine causes a stress reaction and/or a pro-inflammatory response that inhibits the agonistic action of retinoic acid. Moreover, we show that the effect of cigarette smoke on the developing fetus could represent systemic and aggressive impacts in the short term, causing malformations during certain stages of development. Our work provides the first approach describing how different tobacco constituents affect a broad range of biological process in human embryonic development.
Conflict of interest statement
Figures





References
-
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, et al. (2002) Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 21(48): 7435–7451. - PubMed
-
- Fowles J, Bates M (2000). The chemical constituents in cigarette and cigarette smoke: priorities for harm reduction. Available: http://www.moh.govt.nz/moh.nsf/pagescm/1003/File/chemicalconstituentscig.... Accessed 2013 March 26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

