Primary breast cancer tumours contain high amounts of IgA1 immunoglobulin: an immunohistochemical analysis of a possible carrier of the tumour-associated Tn antigen
- PMID: 23637900
- PMCID: PMC3630176
- DOI: 10.1371/journal.pone.0061749
Primary breast cancer tumours contain high amounts of IgA1 immunoglobulin: an immunohistochemical analysis of a possible carrier of the tumour-associated Tn antigen
Abstract
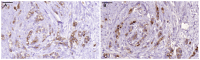
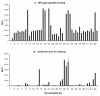
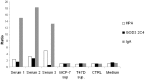
The Tn antigen (GalNAc alpha-O-Ser/Thr) as defined by the binding of the lectin, helix pomatia agglutinin (HPA) or anti-Tn monoclonal antibodies, is known to be exposed in a majority of cancers, and it has also been shown to correlate positively with the metastatic capacity in breast carcinoma. The short O-glycan that forms the antigen is carried by a number of different proteins. One potential carrier of the Tn antigen is immunoglobulin A1 (IgA1), which we surprisingly found in tumour cells of the invasive parts of primary breast carcinoma. Conventional immunohistochemical analysis of paraffin-embedded sections from primary breast cancers showed IgA1 to be present in the cytoplasm and plasma membrane of 35 out of 36 individual primary tumours. The immunohistochemical staining of HPA and anti-Tn antibody (GOD3-2C4) did to some extent overlap with the presence of IgA1 in the tumours, but differences were seen in the percentage of stained cells and in the staining pattern in the different breast cancers analysed. Anti-Tn antibody and HPA were also shown to specifically bind to a number of possible constellations of the Tn antigen in the hinge region of IgA1. Both reagents could also detect the presence of Tn positive IgA in serum. On average 51% of the tumour cells in the individual breast cancer tumour sections showed staining for IgA1. The overall amount of staining in the invasive part of the tumour with the anti Tn antibody was 67%, and 93% with HPA. The intra-expression or uptake of IgA1 in breast cancer makes it a new potential carrier of the tumour associated and immunogenic Tn antigen.
Conflict of interest statement
Figures







References
-
- Ando H, Matsushita T, Masako Wakitani, Takashi Sato, Kodama-Nishida S, et al. (2008) Mouse-Human Chimeric Anti-Tn IgG1 Induced Anti-tumor Activity against Jurkat Cells in Vitro and in Vivo. . Biol Pharm Bull 31: 1739–44. - PubMed
-
- Freire T, Medeiros A, Reis CA, Real FX, Osinaga E (2003) Biochemical characterization of soluble Tn glycoproteins from malignant effusions of patients with carcinomas. Oncol Rep 10: 1577–85. - PubMed
-
- Dwek MV, Ross HA, Streets AJ, Brooks SA, Adam E, et al. (2001) Helix pomatia agglutinin lectin-binding oligosaccharides of aggressive breast cancer. Int J Cancer Mar 20;95 2: 79–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

