Reactive oxygen species-activated nanoprodrug of Ibuprofen for targeting traumatic brain injury in mice
- PMID: 23637912
- PMCID: PMC3634829
- DOI: 10.1371/journal.pone.0061819
Reactive oxygen species-activated nanoprodrug of Ibuprofen for targeting traumatic brain injury in mice
Abstract
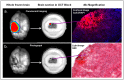
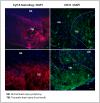
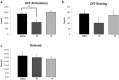
Traumatic brain injury (TBI) is an enormous public health problem, with 1.7 million new cases of TBI recorded annually by the Centers for Disease Control. However, TBI has proven to be an extremely challenging condition to treat. Here, we apply a nanoprodrug strategy in a mouse model of TBI. The novel nanoprodrug contains a derivative of the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen in an emulsion with the antioxidant α-tocopherol. The ibuprofen derivative, Ibu2TEG, contains a tetra ethylene glycol (TEG) spacer consisting of biodegradable ester bonds. The biodegradable ester bonds ensure that the prodrug molecules break down hydrolytically or enzymatically. The drug is labeled with the fluorescent reporter Cy5.5 using nonbiodegradable bonds to 1-octadecanethiol, allowing us to reliably track its accumulation in the brain after TBI. We delivered a moderate injury using a highly reproducible mouse model of closed-skull controlled cortical impact to the parietal region of the cortex, followed by an injection of the nanoprodrug at a dose of 0.2 mg per mouse. The blood brain barrier is known to exhibit increased permeability at the site of injury. We tested for accumulation of the fluorescent drug particles at the site of injury using confocal and bioluminescence imaging of whole brains and brain slices 36 hours after administration. We demonstrated that the drug does accumulate preferentially in the region of injured tissue, likely due to an enhanced permeability and retention (EPR) phenomenon. The use of a nanoprodrug approach to deliver therapeutics in TBI represents a promising potential therapeutic modality.
Conflict of interest statement
Figures
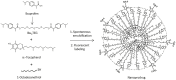


Similar articles
-
Reactive oxygen species responsive nanoprodrug to treat intracranial glioblastoma.ACS Nano. 2013 Apr 23;7(4):3061-77. doi: 10.1021/nn400347j. Epub 2013 Apr 4. ACS Nano. 2013. PMID: 23557138
-
Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats.Exp Neurol. 2006 Oct;201(2):301-7. doi: 10.1016/j.expneurol.2006.04.008. Epub 2006 Jun 9. Exp Neurol. 2006. PMID: 16764859
-
Ibuprofen attenuates the inflammatory response and allows formation of migratory neuroblasts from grafted stem cells after traumatic brain injury.Restor Neurol Neurosci. 2012;30(1):9-19. doi: 10.3233/RNN-2011-0606. Restor Neurol Neurosci. 2012. PMID: 22377906
-
Dual-targeting for brain-specific drug delivery: synthesis and biological evaluation.Drug Deliv. 2018 Nov;25(1):426-434. doi: 10.1080/10717544.2018.1431978. Drug Deliv. 2018. PMID: 29382239 Free PMC article.
-
Alkyl ester prodrugs for improved topical delivery of ibuprofen.Indian J Exp Biol. 2001 Mar;39(3):280-3. Indian J Exp Biol. 2001. PMID: 11495290
Cited by
-
Temporal assessment of nanoparticle accumulation after experimental brain injury: Effect of particle size.Sci Rep. 2016 Jul 22;6:29988. doi: 10.1038/srep29988. Sci Rep. 2016. PMID: 27444615 Free PMC article.
-
Blood-brainbarrier disruption dictates nanoparticle accumulation following experimental brain injury.Nanomedicine. 2018 Oct;14(7):2155-2166. doi: 10.1016/j.nano.2018.06.004. Epub 2018 Jun 19. Nanomedicine. 2018. PMID: 29933022 Free PMC article.
-
Exploring hydrogen peroxide responsive thiazolidinone-based prodrugs.Chem Commun (Camb). 2015 Apr 28;51(33):7116-9. doi: 10.1039/c4cc09921d. Chem Commun (Camb). 2015. PMID: 25811298 Free PMC article.
-
Localization of Multi-Lamellar Vesicle Nanoparticles to Injured Brain Tissue in a Controlled Cortical Impact Injury Model of Traumatic Brain Injury in Rodents.Neurotrauma Rep. 2022 Apr 5;3(1):158-167. doi: 10.1089/neur.2021.0049. eCollection 2022. Neurotrauma Rep. 2022. PMID: 35403102 Free PMC article.
-
Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders.Int J Mol Sci. 2020 Aug 23;21(17):6070. doi: 10.3390/ijms21176070. Int J Mol Sci. 2020. PMID: 32842530 Free PMC article. Review.
References
-
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL (2006) Incidence of traumatic brain injury in the United States, 2003. The Journal of Head Trauma Rehabilitation 21: 544–548. - PubMed
-
- Summers CR, Ivins B, Schwab KA (2009) Traumatic Brain Injury in the United States: An Epidemiologic Overview. Mount Sinai Journal of Medicine 76: 105–110. - PubMed
-
- Xu J, Kochanek KD, Murphy SL, Tejada-Vera B, others (n.d.) Deaths: final data for 2007. National Vital Statistics Reports 58: 19. - PubMed
-
- WISQARS Years of Potential Life Lost (YPLL) Reports, 1999–2007 (n.d.). National Center for Injury Prevention and Control. Available:http://webappa.cdc.gov/sasweb/ncipc/ypll10.html.
-
- Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

