2009 A(H1N1) seroconversion rates and risk factors among the general population in Vientiane Capital, Laos
- PMID: 23637928
- PMCID: PMC3630132
- DOI: 10.1371/journal.pone.0061909
2009 A(H1N1) seroconversion rates and risk factors among the general population in Vientiane Capital, Laos
Abstract
Objective: To assess 2009 A(H1N1) seroconversion rates and their determinants within an unvaccinated population in Vientiane Capital, Laos.
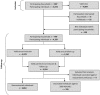
Methods: CoPanFlu Laos, a general population cohort of 807 households and 4,072 participants was established in March 2010. Sociodemographic data, epidemiological data, and capillary blood samples were collected from all the household members in March, and again in October 2010, in order to assess the level of antibodies to 2009 A(H1N1) with the haemagglutination inhibition assay. 2009 A(H1N1) seroconversion was defined as a fourfold or greater increase in titre between inclusion and follow-up. Determinants for pandemic influenza infection were studied using the generalized estimating equations model, taking household clustering into account.
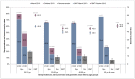
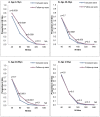
Results: Between March and November 2010, 3,524 paired sera were tested. Prior to the pandemic, our cohort was almost completely vaccine-naive for seasonal influenza. The overall seroconversion rate among nonvaccinated individuals (n = 2,810) was 14.3% (95%CI [13.0, 15.6]), with the highest rate for participants under 20 yo (19.8%, 95%CI [17.4, 22.4]) and the lowest rate for participants over 60 yo (6.5%, 95%CI [3.7, 10.4]). Participants with lower baseline titres had significantly higher infection rates, with a dose-effect relationship. Odds ratios (ORs) ranged from 76.5 (95%CI [27.1, 215.8]), for those with a titre at inclusion of 1∶10, to 8.1 (95%CI [3.3, 20.4]), for those with a titre of 1∶40. Having another household member with a titre ≥1∶80 was associated with a higher likelihood of immunity (OR = 3.3, 95%CI [2.8, 3.9]).
Conclusion: The determinants and age distribution for seroconversion within a vaccine-naive population were similar to those found in developed countries. This pandemic was characterized by strong epidemiological determinants, regardless of geographical zone and level of development. Moreover, we detected pre-existing cross-reacting antibodies in participants over 60 yo, which could not have originated from former multiple vaccination as has been suggested elsewhere.
Conflict of interest statement
Figures
Similar articles
-
Absence of cross-reactive antibodies to influenza A (H1N1) 2009 before and after vaccination with 2009 Southern Hemisphere seasonal trivalent influenza vaccine in children aged 6 months-9 years: a prospective study.Influenza Other Respir Viruses. 2011 Jan;5(1):7-11. doi: 10.1111/j.1750-2659.2010.00172.x. Epub 2010 Aug 24. Influenza Other Respir Viruses. 2011. PMID: 21138535 Free PMC article. Clinical Trial.
-
Improved serological response to H1N1 monovalent vaccine associated with viral suppression among HIV-1-infected patients during the 2009 influenza (H1N1) pandemic in the Southern Hemisphere.HIV Med. 2012 Jul;13(6):352-7. doi: 10.1111/j.1468-1293.2011.00987.x. Epub 2012 Feb 2. HIV Med. 2012. PMID: 22296264
-
Determinants of individuals' risks to 2009 pandemic influenza virus infection at household level amongst Djibouti city residents--a CoPanFlu cross-sectional study.Virol J. 2014 Jan 27;11:13. doi: 10.1186/1743-422X-11-13. Virol J. 2014. PMID: 24468218 Free PMC article.
-
Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009.Health Technol Assess. 2010 Dec;14(55):115-92. doi: 10.3310/hta14550-03. Health Technol Assess. 2010. PMID: 21208549
-
An indirect comparison meta-analysis of AS03 and MF59 adjuvants in pandemic influenza A(H1N1)pdm09 vaccines.Vaccine. 2019 Jul 18;37(31):4246-4255. doi: 10.1016/j.vaccine.2019.06.039. Epub 2019 Jun 26. Vaccine. 2019. PMID: 31253447 Review.
Cited by
-
"Epidemiology and aetiology of influenza-like illness among households in metropolitan Vientiane, Lao PDR": A prospective, community-based cohort study.PLoS One. 2019 Apr 5;14(4):e0214207. doi: 10.1371/journal.pone.0214207. eCollection 2019. PLoS One. 2019. PMID: 30951544 Free PMC article.
-
Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study.Lancet Respir Med. 2014 Jun;2(6):445-54. doi: 10.1016/S2213-2600(14)70034-7. Epub 2014 Mar 17. Lancet Respir Med. 2014. PMID: 24717637 Free PMC article.
-
Maternal Influenza Vaccination and the Risk of Laboratory-Confirmed Influenza Among Household Contacts Under the Age of Five in Mali.Am J Trop Med Hyg. 2019 Jan;100(1):159-164. doi: 10.4269/ajtmh.18-0450. Am J Trop Med Hyg. 2019. PMID: 30526742 Free PMC article. Clinical Trial.
-
Determinants of influenza transmission in South East Asia: insights from a household cohort study in Vietnam.PLoS Pathog. 2014 Aug 21;10(8):e1004310. doi: 10.1371/journal.ppat.1004310. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25144780 Free PMC article.
-
Causal analysis of H1N1pdm09 influenza infection risk in a household cohort.J Epidemiol Community Health. 2015 Mar;69(3):272-7. doi: 10.1136/jech-2014-204678. Epub 2014 Nov 21. J Epidemiol Community Health. 2015. PMID: 25416792 Free PMC article.
References
-
- Moura FE (2010) Influenza in the tropics. Current opinion in infectious diseases 23: 415–420. - PubMed
-
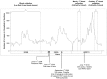
- WHO (2009) Human infections of influenza A (H1N1): Perspective from WHO Lao P.D.R. (update 22). Available: www.unlao.org/H1N1/influenzah1n1.asp. Accessed 20 March 2013.
-
- WHO (2010) Influenza Update: Perspective from WHO Lao P.D.R. (update 40). Available: www.unlao.org/H1N1/influenzah1n1.asp. Accessed 20 March 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

