Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors
- PMID: 23637972
- PMCID: PMC3637363
- DOI: 10.1371/journal.pone.0062097
Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors
Erratum in
- PLoS One. 2013;8(9). doi:10.1371/annotation/99ee1789-a658-4fb0-8593-40a40e9f344a
Abstract
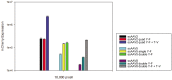
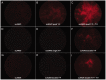
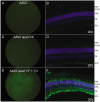
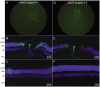
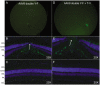
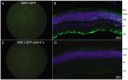
Development of viral vectors capable of transducing photoreceptors by less invasive methods than subretinal injection would provide a major advancement in retinal gene therapy. We sought to develop novel AAV vectors optimized for photoreceptor transduction following intravitreal delivery and to develop methodology for quantifying this transduction in vivo. Surface exposed tyrosine (Y) and threonine (T) residues on the capsids of AAV2, AAV5 and AAV8 were changed to phenylalanine (F) and valine (V), respectively. Transduction efficiencies of self-complimentary, capsid-mutant and unmodified AAV vectors containing the smCBA promoter and mCherry cDNA were initially scored in vitro using a cone photoreceptor cell line. Capsid mutants exhibiting the highest transduction efficiencies relative to unmodified vectors were then injected intravitreally into transgenic mice constitutively expressing a Rhodopsin-GFP fusion protein in rod photoreceptors (Rho-GFP mice). Photoreceptor transduction was quantified by fluorescent activated cell sorting (FACS) by counting cells positive for both GFP and mCherry. To explore the utility of the capsid mutants, standard, (non-self-complementary) AAV vectors containing the human rhodopsin kinase promoter (hGRK1) were made. Vectors were intravitreally injected in wildtype mice to assess whether efficient expression exclusive to photoreceptors was achievable. To restrict off-target expression in cells of the inner and middle retina, subsequent vectors incorporated multiple target sequences for miR181, an miRNA endogenously expressed in the inner and middle retina. Results showed that AAV2 containing four Y to F mutations combined with a single T to V mutation (quadY-F+T-V) transduced photoreceptors most efficiently. Robust photoreceptor expression was mediated by AAV2(quadY-F+T-V) -hGRK1-GFP. Observed off-target expression was reduced by incorporating target sequence for a miRNA highly expressed in inner/middle retina, miR181c. Thus we have identified a novel AAV vector capable of transducing photoreceptors following intravitreal delivery to mouse. Furthermore, we describe a robust methodology for quantifying photoreceptor transduction from intravitreally delivered AAV vectors.
Conflict of interest statement
Figures

References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, et al. (2008) Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 358: 2231–2239. - PubMed
-
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS (2010) Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet 11: 273–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

