A novel beta-defensin antimicrobial peptide in Atlantic cod with stimulatory effect on phagocytic activity
- PMID: 23638029
- PMCID: PMC3636224
- DOI: 10.1371/journal.pone.0062302
A novel beta-defensin antimicrobial peptide in Atlantic cod with stimulatory effect on phagocytic activity
Abstract
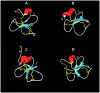
A novel defensin antimicrobial peptide gene was identified in Atlantic cod, Gadus morhua. This three exon/two intron defensin gene codes for a peptide precursor consisting of two domains: a signal peptide of 26 amino acids and a mature peptide of 40 residues. The mature cod defensin has six conserved cysteine residues that form 1-5, 2-4 and 3-6 disulphide bridges. This pattern is typical of beta-defensins and this gene was therefore named cod beta-defensin (defb). The tertiary structure of Defb exhibits an α/β fold with one α helix and β1β2β3 sheets. RT-PCR analysis indicated that defb transcripts were present mainly in the swim bladder and peritoneum wall but could also be detected at moderate to low levels in skin, head- and excretory kidneys. In situ hybridisation revealed that defb was specifically expressed by cells located in the swim bladder submucosa and the oocytes. During embryonic development, defb gene transcripts were detectable from the golden eye stage onwards and their expression was restricted to the swim bladder and retina. Defb was differentially expressed in several tissues following antigenic challenge with Vibrio anguillarum, being up-regulated up to 25-fold in head kidney. Recombinant Defb displayed antibacterial activity, with a minimal inhibitory concentration of 0.4-0.8 µM and 25-50 µM against the Gram-(+) bacteria Planococcus citreus and Micrococcus luteus, respectively. In addition, Defb stimulated phagocytic activity of cod head kidney leucocytes in vitro. These findings imply that beta-defensins may play an important role in the innate immune response of Atlantic cod.
Conflict of interest statement
Figures







References
-
- Taylor K, Barran PE, Dorin JR (2008) Review: Structure-activity relationships in beta-defensin peptides. Biopolymers 90: 1–7. - PubMed
-
- Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3: 710–720. - PubMed
-
- Fernandes JM, Smith VJ (2002) A novel antimicrobial function for a ribosomal peptide from rainbow trout skin. Biochem Biophys Res Commun 296: 167–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

