Differential susceptibility and response of primary human myeloid BDCA1(+) dendritic cells to infection with different Enteroviruses
- PMID: 23638101
- PMCID: PMC3634769
- DOI: 10.1371/journal.pone.0062502
Differential susceptibility and response of primary human myeloid BDCA1(+) dendritic cells to infection with different Enteroviruses
Abstract
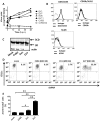
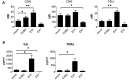
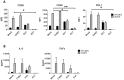
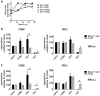
Coxsackie B viruses (CVBs) and echoviruses (EVs) form the Human Enterovirus-B (HEV-B) species within the family Picornaviridae. HEV-B infections are widespread and generally cause mild disease; however, severe infections occur and HEV-B are associated with various chronic diseases such as cardiomyopathy and type 1 diabetes. Dendritic cells (DCs) are the professional antigen-presenting cells of our immune system and initiate and control immune responses to invading pathogens, yet also maintain tolerance to self-antigens. We previously reported that EVs, but not CVBs, can productively infect in vitro generated monocyte-derived DCs. The interactions between HEV-B and human myeloid DCs (mDCs) freshly isolated from blood, however, remain unknown. Here, we studied the susceptibility and responses of BDCA1(+) mDC to HEV-B species and found that these mDC are susceptible to EV, but not CVB infection. Productive EV7 infection resulted in massive, rapid cell death without DC activation. Contrary, EV1 infection, which resulted in lower virus input at the same MOI, resulted in DC activation as observed by production of type I interferon-stimulated genes (ISGs), upregulation of co-stimulatory and co-inhibitory molecules (CD80, CD86, PDL1) and production of IL-6 and TNF-α, with a relative moderate decrease in cell viability. EV1-induced ISG expression depended on virus replication. CVB infection did not affect DC viability and resulted in poor induction of ISGs and CD80 induction in part of the donors. These data show for the first time the interaction between HEV-B species and BDCA1(+) mDCs isolated freshly from blood. Our data indicate that different HEV-B species can influence DC homeostasis in various ways, possibly contributing to HEV-B associated pathology.
Conflict of interest statement
Figures

Similar articles
-
Enterovirus-infected β-cells induce distinct response patterns in BDCA1+ and BDCA3+ human dendritic cells.PLoS One. 2015 Mar 25;10(3):e0121670. doi: 10.1371/journal.pone.0121670. eCollection 2015. PLoS One. 2015. PMID: 25806537 Free PMC article.
-
Hepatitis B Virus Surface Antigen Activates Myeloid Dendritic Cells via a Soluble CD14-Dependent Mechanism.J Virol. 2016 Jun 24;90(14):6187-6199. doi: 10.1128/JVI.02903-15. Print 2016 Jul 15. J Virol. 2016. PMID: 27099316 Free PMC article.
-
Echovirus infection causes rapid loss-of-function and cell death in human dendritic cells.Cell Microbiol. 2007 Jun;9(6):1507-18. doi: 10.1111/j.1462-5822.2007.00888.x. Epub 2007 Feb 9. Cell Microbiol. 2007. PMID: 17298395
-
Myeloid dendritic cells: Development, functions, and role in atherosclerotic inflammation.Immunobiology. 2015 Jun;220(6):833-44. doi: 10.1016/j.imbio.2014.12.010. Epub 2015 Jan 5. Immunobiology. 2015. PMID: 25595536 Review.
-
Crosstalk between type I and II interferons in regulation of myeloid cell responses during bacterial infection.Curr Opin Immunol. 2018 Oct;54:35-41. doi: 10.1016/j.coi.2018.05.014. Epub 2018 Jun 7. Curr Opin Immunol. 2018. PMID: 29886270 Free PMC article. Review.
Cited by
-
Enterovirus-infected β-cells induce distinct response patterns in BDCA1+ and BDCA3+ human dendritic cells.PLoS One. 2015 Mar 25;10(3):e0121670. doi: 10.1371/journal.pone.0121670. eCollection 2015. PLoS One. 2015. PMID: 25806537 Free PMC article.
-
PRMT5 inhibition modulates murine dendritic cells activation by inhibiting the metabolism switch: a new therapeutic target in periodontitis.Ann Transl Med. 2021 May;9(9):755. doi: 10.21037/atm-20-7362. Ann Transl Med. 2021. PMID: 34268368 Free PMC article.
-
Enterovirus-71 virus-like particles induce the activation and maturation of human monocyte-derived dendritic cells through TLR4 signaling.PLoS One. 2014 Oct 31;9(10):e111496. doi: 10.1371/journal.pone.0111496. eCollection 2014. PLoS One. 2014. PMID: 25360749 Free PMC article.
-
Distinct activation of primary human BDCA1(+) dendritic cells upon interaction with stressed or infected β cells.Clin Exp Immunol. 2016 Jun;184(3):293-307. doi: 10.1111/cei.12779. Epub 2016 Mar 23. Clin Exp Immunol. 2016. PMID: 26888163 Free PMC article.
-
Coxsackievirus B4 Can Infect Human Peripheral Blood-Derived Macrophages.Viruses. 2015 Nov 24;7(11):6067-79. doi: 10.3390/v7112924. Viruses. 2015. PMID: 26610550 Free PMC article.
References
-
- Sporri R, Reis e Sousa C (2005) Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol 6: 163–170. - PubMed
-
- Arrode G, Davrinche C (2003) Dendritic cells and HCMV cross-presentation. Curr Top Microbiol Immunol 276: 277–294. - PubMed
-
- Ruedl C, Storni T, Lechner F, Bachi T, Bachmann MF (2002) Cross-presentation of virus-like particles by skin-derived CD8(-) dendritic cells: a dispensable role for TAP. Eur J Immunol 32: 818–825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

