MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus
- PMID: 23638108
- PMCID: PMC3637215
- DOI: 10.1371/journal.pone.0062532
MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus
Abstract
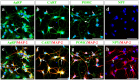
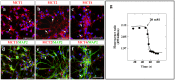
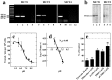
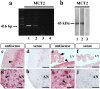
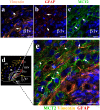
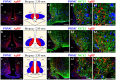
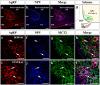
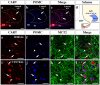
Hypothalamic neurons of the arcuate nucleus control food intake, releasing orexigenic and anorexigenic neuropeptides in response to changes in glucose concentration. Several studies have suggested that the glucosensing mechanism is governed by a metabolic interaction between neurons and glial cells via lactate flux through monocarboxylate transporters (MCTs). Hypothalamic glial cells (tanycytes) release lactate through MCT1 and MCT4; however, similar analyses in neuroendocrine neurons have yet to be undertaken. Using primary rat hypothalamic cell cultures and fluorimetric assays, lactate incorporation was detected. Furthermore, the expression and function of MCT2 was demonstrated in the hypothalamic neuronal cell line, GT1-7, using kinetic and inhibition assays. Moreover, MCT2 expression and localization in the Sprague Dawley rat hypothalamus was analyzed using RT-PCR, in situ hybridization and Western blot analyses. Confocal immunohistochemistry analyses revealed MCT2 localization in neuronal but not glial cells. Moreover, MCT2 was localized to ∼90% of orexigenic and ~60% of anorexigenic neurons as determined by immunolocalization analysis of AgRP and POMC with MCT2-positives neurons. Thus, MCT2 distribution coupled with lactate uptake by hypothalamic neurons suggests that hypothalamic neurons control food intake using lactate to reflect changes in glucose levels.
Conflict of interest statement
Figures



References
-
- Oomura Y, Yoshimatsu H (1984) Neural network of glucose monitoring system. J Auton Nerv Syst 10: 359–372. - PubMed
-
- Yang XJ, Kow LM, Funabashi T, Mobbs CV (1999) Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes 48: 1763–1772. - PubMed
-
- Blouet C, Schwartz GJ (2010) Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 209: 1–12. - PubMed
-
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404: 661–671. - PubMed
-
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA (2004) Neuronal glucosensing: what do we know after 50 years? Diabetes 53: 2521–2528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

