Hepatitis C virus NS2 protein inhibits DNA damage pathway by sequestering p53 to the cytoplasm
- PMID: 23638118
- PMCID: PMC3640050
- DOI: 10.1371/journal.pone.0062581
Hepatitis C virus NS2 protein inhibits DNA damage pathway by sequestering p53 to the cytoplasm
Abstract
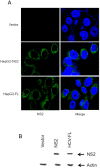
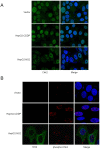
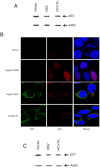
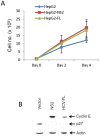
Chronic hepatitis C virus (HCV) infection is an important cause of morbidity and mortality globally, and often leads to end-stage liver disease. The DNA damage checkpoint pathway induces cell cycle arrest for repairing DNA in response to DNA damage. HCV infection has been involved in this pathway. In this study, we assess the effects of HCV NS2 on DNA damage checkpoint pathway. We have observed that HCV NS2 induces ataxia-telangiectasia mutated checkpoint pathway by inducing Chk2, however, fails to activate the subsequent downstream pathway. Further study suggested that p53 is retained in the cytoplasm of HCV NS2 expressing cells, and p21 expression is not enhanced. We further observed that HCV NS2 expressing cells induce cyclin E expression and promote cell growth. Together these results suggested that HCV NS2 inhibits DNA damage response by altering the localization of p53, and may play a role in the pathogenesis of HCV infection.
Conflict of interest statement
Figures

Similar articles
-
HCV NS2 protein inhibits cell proliferation and induces cell cycle arrest in the S-phase in mammalian cells through down-regulation of cyclin A expression.Virus Res. 2006 Nov;121(2):134-43. doi: 10.1016/j.virusres.2006.02.004. Epub 2006 Jun 23. Virus Res. 2006. PMID: 16797769
-
Hepatitis C virus core protein inhibits hepatitis B virus replication by downregulating HBx levels via Siah-1-mediated proteasomal degradation during coinfection.J Gen Virol. 2021 Dec;102(12). doi: 10.1099/jgv.0.001701. J Gen Virol. 2021. PMID: 34882535
-
Sterigmatocystin-induced DNA damage triggers G2 arrest via an ATM/p53-related pathway in human gastric epithelium GES-1 cells in vitro.PLoS One. 2013 May 21;8(5):e65044. doi: 10.1371/journal.pone.0065044. Print 2013. PLoS One. 2013. PMID: 23705030 Free PMC article.
-
Hepatitis C Virus Indirectly Disrupts DNA Damage-Induced p53 Responses by Activating Protein Kinase R.mBio. 2017 Apr 25;8(2):e00121-17. doi: 10.1128/mBio.00121-17. mBio. 2017. PMID: 28442604 Free PMC article.
-
DNA damage checkpoint kinases in cancer.Expert Rev Mol Med. 2020 Jun 8;22:e2. doi: 10.1017/erm.2020.3. Expert Rev Mol Med. 2020. PMID: 32508294 Review.
Cited by
-
MHC Phosphopeptides: Promising Targets for Immunotherapy of Cancer and Other Chronic Diseases.Mol Cell Proteomics. 2021;20:100112. doi: 10.1016/j.mcpro.2021.100112. Epub 2021 Jun 12. Mol Cell Proteomics. 2021. PMID: 34129940 Free PMC article. Review.
-
Activation of the DNA Damage Response by RNA Viruses.Biomolecules. 2016 Jan 6;6(1):2. doi: 10.3390/biom6010002. Biomolecules. 2016. PMID: 26751489 Free PMC article. Review.
-
Classical swine fever virus Shimen infection increases p53 signaling to promote cell cycle arrest in porcine alveolar macrophages.Oncotarget. 2017 Jul 5;8(34):55938-55949. doi: 10.18632/oncotarget.18997. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915564 Free PMC article.
-
[Hepatitis B and C: mechanisms of virus-induced liver pathogenesis and tumorigenesis].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022 Feb;65(2):228-237. doi: 10.1007/s00103-021-03482-y. Epub 2022 Jan 11. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022. PMID: 35015106 Free PMC article. Review. German.
-
Molecular basis of hepatocellular carcinoma induced by hepatitis C virus infection.World J Hepatol. 2017 Dec 28;9(36):1305-1314. doi: 10.4254/wjh.v9.i36.1305. World J Hepatol. 2017. PMID: 29359013 Free PMC article. Review.
References
-
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, et al. (1989) Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244: 359–362. - PubMed
-
- Kuiken C, Simmonds P (2009) Nomenclature and numbering of the hepatitis C virus. Methods Mol Biol 510: 33–53. - PubMed
-
- Zein NN, Rakela J, Krawitt EL, Reddy KR, Tominaga T, et al. (1996) Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Collaborative Study Group". Ann Intern Med 125: 634–639. - PubMed
-
- Jin DY (2007) Molecular pathogenesis of hepatitis C virus-associated hepatocellular carcinoma. Front Biosci 12: 222–233. - PubMed
-
- Koike K (2007) Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol 22: S108–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

