Molecular modeling of the human hemoglobin-haptoglobin complex sheds light on the protective mechanisms of haptoglobin
- PMID: 23638175
- PMCID: PMC3637213
- DOI: 10.1371/journal.pone.0062996
Molecular modeling of the human hemoglobin-haptoglobin complex sheds light on the protective mechanisms of haptoglobin
Abstract
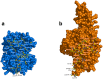
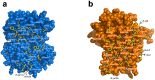
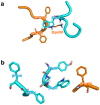
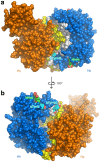
Hemoglobin (Hb) plays a critical role in human physiological function by transporting O2. Hb is safe and inert within the confinement of the red blood cell but becomes reactive and toxic upon hemolysis. Haptoglobin (Hp) is an acute-phase serum protein that scavenges Hb and the resulting Hb-Hp complex is subjected to CD163-mediated endocytosis by macrophages. The interaction between Hb and Hp is extraordinarily strong and largely irreversible. As the structural details of the human Hb-Hp complex are not yet available, this study reports for the first time on insights of the binding modalities and molecular details of the human Hb-Hp interaction by means of protein-protein docking. Furthermore, residues that are pertinent for complex formation were identified by computational alanine scanning mutagenesis. Results revealed that the surface of the binding interface of Hb-Hp is not flat and protrudes into each binding partner. It was also observed that the secondary structures at the Hb-Hp interface are oriented as coils and α-helices. When dissecting the interface in more detail, it is obvious that several tyrosine residues of Hb, particularly β145Tyr, α42Tyr and α140Tyr, are buried in the complex and protected from further oxidative reactions. Such finding opens up new avenues for the design of Hp mimics which may be used as alternative clinical Hb scavengers.
Conflict of interest statement
Figures
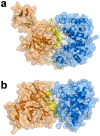
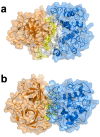
Similar articles
-
Spin trapping combined with quantitative mass spectrometry defines free radical redistribution within the oxidized hemoglobin:haptoglobin complex.Free Radic Biol Med. 2015 Aug;85:259-68. doi: 10.1016/j.freeradbiomed.2015.04.023. Epub 2015 Apr 28. Free Radic Biol Med. 2015. PMID: 25933590
-
Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification.Blood. 2009 Mar 12;113(11):2578-86. doi: 10.1182/blood-2008-08-174466. Epub 2009 Jan 8. Blood. 2009. PMID: 19131549
-
Interactions of Haptoglobin with Monomeric Globin Species: Insights from Molecular Modeling and Native Electrospray Ionization Mass Spectrometry.Biochemistry. 2016 Mar 29;55(12):1918-28. doi: 10.1021/acs.biochem.5b00807. Epub 2016 Mar 17. Biochemistry. 2016. PMID: 26937685
-
Trapping of human hemoglobin by haptoglobin: molecular mechanisms and clinical applications.Antioxid Redox Signal. 2013 Jun 10;18(17):2364-74. doi: 10.1089/ars.2012.4878. Epub 2012 Oct 18. Antioxid Redox Signal. 2013. PMID: 22900934 Review.
-
Haptoglobin: From hemoglobin scavenging to human health.Mol Aspects Med. 2020 Jun;73:100851. doi: 10.1016/j.mam.2020.100851. Epub 2020 Jul 11. Mol Aspects Med. 2020. PMID: 32660714 Review.
Cited by
-
Babesia microti-induced fulminant sepsis in an immunocompromised host: A case report and the case-specific literature review.Open Life Sci. 2022 Sep 14;17(1):1200-1207. doi: 10.1515/biol-2022-0448. eCollection 2022. Open Life Sci. 2022. PMID: 36185407 Free PMC article.
-
SMOC2, OGN, FCN3, and SERPINA3 could be biomarkers for the evaluation of acute decompensated heart failure caused by venous congestion.Front Cardiovasc Med. 2024 Dec 9;11:1406662. doi: 10.3389/fcvm.2024.1406662. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39717447 Free PMC article.
-
Haptoglobin as a Biomarker.Biochem Mosc Suppl B Biomed Chem. 2021;15(3):184-198. doi: 10.1134/S1990750821030069. Epub 2021 Aug 16. Biochem Mosc Suppl B Biomed Chem. 2021. PMID: 34422226 Free PMC article.
-
Role of Haptoglobin in Health and Disease: A Focus on Diabetes.Clin Diabetes. 2016 Jul;34(3):148-57. doi: 10.2337/diaclin.34.3.148. Clin Diabetes. 2016. PMID: 27621532 Free PMC article.
-
Haptoglobin Is a Divergent MASP Family Member That Neofunctionalized To Recycle Hemoglobin via CD163 in Mammals.J Immunol. 2018 Oct 15;201(8):2483-2491. doi: 10.4049/jimmunol.1800508. Epub 2018 Sep 7. J Immunol. 2018. PMID: 30194112 Free PMC article.
References
-
- Vinogradov SN, Moens L (2008) Diversity of globin function: enzymatic, transport, storage, and sensing. J Biol Chem 283: 8773–8777. - PubMed
-
- Reeder BJ (2010) The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal 13: 1087–1123. - PubMed
-
- Olsson MG, Allhorn M, Bulow L, Hansson SR, Ley D, et al. (2012) Pathological conditions involving extracellular hemoglobin: molecular mechanisms, clinical significance, and novel therapeutic opportunities for alpha(1)-microglobulin. Antioxid Redox Signal 17: 813–846. - PubMed
-
- Rother RP, Bell L, Hillmen P, Gladwin MT (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. J Am Med Assoc 293: 1653–1662. - PubMed
-
- Polonovski M, Jayle MF (1938) Existence dans le plasma sanguin d'une substance activant l'action peroxydasique de l'hemoglobine. C R Acad Sc (Paris) 129: 457.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

