Single-reaction, multiplex, real-time rt-PCR for the detection, quantitation, and serotyping of dengue viruses
- PMID: 23638191
- PMCID: PMC3630127
- DOI: 10.1371/journal.pntd.0002116
Single-reaction, multiplex, real-time rt-PCR for the detection, quantitation, and serotyping of dengue viruses
Abstract
Background: Dengue fever results from infection with one or more of four different serotypes of dengue virus (DENV). Despite the widespread nature of this infection, available molecular diagnostics have significant limitations. The aim of this study was to develop a multiplex, real-time, reverse transcriptase-PCR (rRT-PCR) for the detection, quantitation, and serotyping of dengue viruses in a single reaction.
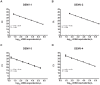
Methodology/principal findings: An rRT-PCR assay targeting the 5' untranslated region and capsid gene of the DENV genome was designed using molecular beacons to provide serotype specificity. Using reference DENV strains, the assay was linear from 7.0 to 1.0 log₁₀ cDNA equivalents/µL for each serotype. The lower limit of detection using genomic RNA was 0.3, 13.8, 0.8, and 12.4 cDNA equivalents/µL for serotypes 1-4, respectively, which was 6- to 275-fold more analytically sensitive than a widely used hemi-nested RT-PCR. Using samples from Nicaragua collected within the first five days of illness, the multiplex rRT-PCR was positive in 100% (69/69) of specimens that were positive by the hemi-nested assay, with full serotype agreement. Furthermore, the multiplex rRT-PCR detected DENV RNA in 97.2% (35/36) of specimens from Sri Lanka positive for anti-DENV IgM antibodies compared to just 44.4% (16/36) by the hemi-nested RT-PCR. No amplification was observed in 80 clinical samples sent for routine quantitative hepatitis C virus testing or when genomic RNA from other flaviviruses was tested.
Conclusions/significance: This single-reaction, quantitative, multiplex rRT-PCR for DENV serotyping demonstrates superior analytical and clinical performance, as well as simpler workflow compared to the hemi-nested RT-PCR reference. In particular, this multiplex rRT-PCR detects viral RNA and provides serotype information in specimens collected more than five days after fever onset and from patients who had already developed anti-DENV IgM antibodies. The implementation of this assay in dengue-endemic areas has the potential to improve both dengue diagnosis and epidemiologic surveillance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. France: WHO Press. - PubMed
-
- WHO (1997) Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. Geneva: World Health Organization.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources