Transcriptional profiling of the circulating immune response to lassa virus in an aerosol model of exposure
- PMID: 23638192
- PMCID: PMC3636129
- DOI: 10.1371/journal.pntd.0002171
Transcriptional profiling of the circulating immune response to lassa virus in an aerosol model of exposure
Abstract
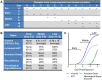
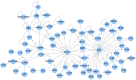
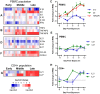
Lassa virus (LASV) is a significant human pathogen that is endemic to several countries in West Africa. Infection with LASV leads to the development of hemorrhagic fever in a significant number of cases, and it is estimated that thousands die each year from the disease. Little is known about the complex immune mechanisms governing the response to LASV or the genetic determinants of susceptibility and resistance to infection. In the study presented here, we have used a whole-genome, microarray-based approach to determine the temporal host response in the peripheral blood mononuclear cells (PBMCs) of non-human primates (NHP) following aerosol exposure to LASV. Sequential sampling over the entire disease course showed that there are strong transcriptional changes of the immune response to LASV exposure, including the early induction of interferon-responsive genes and Toll-like receptor signaling pathways. However, this increase in early innate responses was coupled with a lack of pro-inflammatory cytokine response in LASV exposed NHPs. There was a distinct lack of cytokines such as IL1β and IL23α, while immunosuppressive cytokines such as IL27 and IL6 were upregulated. Comparison of IRF/STAT1-stimulated gene expression with the viral load in LASV exposed NHPs suggests that mRNA expression significantly precedes viremia, and thus might be used for early diagnostics of the disease. Our results provide a transcriptomic survey of the circulating immune response to hemorrhagic LASV exposure and provide a foundation for biomarker identification to allow clinical diagnosis of LASV infection through analysis of the host response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES (1987) A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis 155: 437–444. - PubMed
-
- Monath TP, Newhouse VF, Kemp GE, Setzer HW, Cacciapuoti A (1974) Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science 185: 263–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

