Phage therapy is effective against infection by Mycobacterium ulcerans in a murine footpad model
- PMID: 23638204
- PMCID: PMC3636042
- DOI: 10.1371/journal.pntd.0002183
Phage therapy is effective against infection by Mycobacterium ulcerans in a murine footpad model
Abstract
Background: Buruli Ulcer (BU) is a neglected, necrotizing skin disease caused by Mycobacterium ulcerans. Currently, there is no vaccine against M. ulcerans infection. Although the World Health Organization recommends a combination of rifampicin and streptomycin for the treatment of BU, clinical management of advanced stages is still based on the surgical resection of infected skin. The use of bacteriophages for the control of bacterial infections has been considered as an alternative or to be used in association with antibiotherapy. Additionally, the mycobacteriophage D29 has previously been shown to display lytic activity against M. ulcerans isolates.
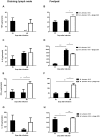
Methodology/principal findings: We used the mouse footpad model of M. ulcerans infection to evaluate the therapeutic efficacy of treatment with mycobacteriophage D29. Analyses of macroscopic lesions, bacterial burdens, histology and cytokine production were performed in both M. ulcerans-infected footpads and draining lymph nodes (DLN). We have demonstrated that a single subcutaneous injection of the mycobacteriophage D29, administered 33 days after bacterial challenge, was sufficient to decrease pathology and to prevent ulceration. This protection resulted in a significant reduction of M. ulcerans numbers accompanied by an increase of cytokine levels (including IFN-γ), both in footpads and DLN. Additionally, mycobacteriophage D29 treatment induced a cellular infiltrate of a lymphocytic/macrophagic profile.
Conclusions/significance: Our observations demonstrate the potential of phage therapy against M. ulcerans infection, paving the way for future studies aiming at the development of novel phage-related therapeutic approaches against BU.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Local and regional re-establishment of cellular immunity during curative antibiotherapy of murine Mycobacterium ulcerans infection.PLoS One. 2012;7(2):e32740. doi: 10.1371/journal.pone.0032740. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22393444 Free PMC article.
-
Antimicrobial activity of Mycobacteriophage D29 Lysin B during Mycobacterium ulcerans infection.PLoS Negl Trop Dis. 2019 Aug 19;13(8):e0007113. doi: 10.1371/journal.pntd.0007113. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31425525 Free PMC article.
-
Cellular immunity confers transient protection in experimental Buruli ulcer following BCG or mycolactone-negative Mycobacterium ulcerans vaccination.PLoS One. 2012;7(3):e33406. doi: 10.1371/journal.pone.0033406. Epub 2012 Mar 8. PLoS One. 2012. PMID: 22413022 Free PMC article.
-
Buruli ulcer: Advances in understanding Mycobacterium ulcerans infection.Dermatol Clin. 2011 Jan;29(1):1-8. doi: 10.1016/j.det.2010.09.006. Dermatol Clin. 2011. PMID: 21095521 Review.
-
Buruli ulcer (Mycobacterium ulcerans infection).Trans R Soc Trop Med Hyg. 2008 Oct;102(10):969-78. doi: 10.1016/j.trstmh.2008.06.006. Epub 2008 Jul 26. Trans R Soc Trop Med Hyg. 2008. PMID: 18657836 Review.
Cited by
-
Vaccine-Specific Immune Responses against Mycobacterium ulcerans Infection in a Low-Dose Murine Challenge Model.Infect Immun. 2020 Feb 20;88(3):e00753-19. doi: 10.1128/IAI.00753-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31818964 Free PMC article.
-
Mycobacteriophages: From Petri dish to patient.PLoS Pathog. 2022 Jul 7;18(7):e1010602. doi: 10.1371/journal.ppat.1010602. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35797343 Free PMC article. Review.
-
Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus.Nat Med. 2019 May;25(5):730-733. doi: 10.1038/s41591-019-0437-z. Epub 2019 May 8. Nat Med. 2019. PMID: 31068712 Free PMC article.
-
High antibody titres induced by protein subunit vaccines using Mycobacterium ulcerans antigens Hsp18 and MUL_3720 with a TLR-2 agonist fail to protect against Buruli ulcer in mice.PeerJ. 2020 Aug 7;8:e9659. doi: 10.7717/peerj.9659. eCollection 2020. PeerJ. 2020. PMID: 32844063 Free PMC article.
-
Bacteriophages as Alternatives to Antibiotics in Clinical Care.Antibiotics (Basel). 2019 Sep 4;8(3):138. doi: 10.3390/antibiotics8030138. Antibiotics (Basel). 2019. PMID: 31487893 Free PMC article. Review.
References
-
- Portaels F, Silva MT, Meyers WM (2009) Buruli ulcer. Clin Dermatol 27: 291–305. - PubMed
-
- Walsh DS, Portaels F, Meyers WM (2011) Buruli ulcer: Advances in understanding Mycobacterium ulcerans infection. Dermatol Clin 29: 1–8. - PubMed
-
- Hong H, Spencer JB, Porter JL, Leadlay PF, Stinear T (2005) A novel mycolactone from a clinical isolate of Mycobacterium ulcerans provides evidence for additional toxin heterogeneity as a result of specific changes in the modular polyketide synthase. Chem Bio Chem 6: 643–648. - PubMed
-
- George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, et al. (1999) Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283: 854–857. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

