A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts
- PMID: 23638389
- PMCID: PMC3628613
- DOI: 10.7717/peerj.51
A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts
Erratum in
-
Correction: A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts.PeerJ. 2013 Mar 20;1:e51/correction-1369083834. eCollection 2013. PeerJ. 2013. PMID: 24191232 Free PMC article.
Abstract
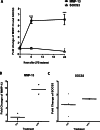
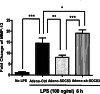
Suppressor of cytokine signaling 3 (SOCS3) is a key regulator of cytokine signaling in macrophages and T cells. Although SOCS3 seems to contribute to the balance between the pro-inflammatory actions of IL-6 family of cytokines and anti-inflammatory signaling of IL-10 by negatively regulating gp130/Jak/Stat3 signal transduction, how and the molecular mechanisms whereby SOCS3 controls the downstream impact of TLR4 are largely unknown and current data are controversial. Furthermore, very little is known regarding SOCS3 function in cells other than myeloid cells and T cells. Our previous study demonstrates that SOCS3 is expressed in osteoblasts and functions as a critical inhibitor of LPS-induced IL-6 expression. However, the function of SOCS3 in osteoblasts remains largely unknown. In the current study, we report for the first time that LPS stimulation of osteoblasts induces the transcriptional activation of matrix metalloproteinase (MMP)-13, a central regulator of bone resorption. Importantly, we demonstrate that SOCS3 overexpression leads to a significant decrease of LPS-induced MMP-13 expression in both primary murine calvariae osteoblasts and a mouse osteoblast-like cell line, MC3T3-E1. Our findings implicate SOCS3 as an important regulatory mediator in bone inflammatory diseases by targeting MMP-13.
Keywords: Cytokine; Inflammation; Osteoblasts; Periodontitis.
Figures



References
-
- Cassatella MA, Gasperini S, Bovolenta C, Calzetti F, Vollebregt M, Scapini P, Marchi M, Suzuki R, Suzuki A, Yoshimura A. Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils: evidence for an IL-10-induced pathway that is independent of STAT protein activation. Blood. 1999;94:2880–2889. - PubMed
-
- Croker BA, Kiu H, Pellegrini M, Toe J, Preston S, Metcalf D, O’Donnell JA, Cengia LH, McArthur K, Nicola NA, Alexander WS, Roberts AW. IL-6 promotes acute and chronic inflammatory disease in the absence of SOCS3. Immunology and Cell Biology. 2012;90:124–129. doi: 10.1038/icb.2011.29. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

