Pulmonary anatomy in the Nile crocodile and the evolution of unidirectional airflow in Archosauria
- PMID: 23638399
- PMCID: PMC3628916
- DOI: 10.7717/peerj.60
Pulmonary anatomy in the Nile crocodile and the evolution of unidirectional airflow in Archosauria
Abstract
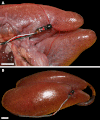
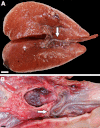
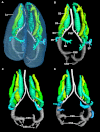
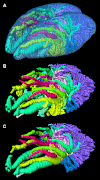
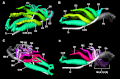
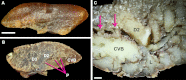
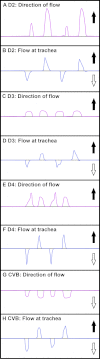
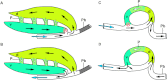
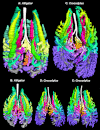
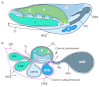
The lungs of birds have long been known to move air in only one direction during both inspiration and expiration through most of the tubular gas-exchanging bronchi (parabronchi). Recently a similar pattern of airflow has been observed in American alligators, a sister taxon to birds. The pattern of flow appears to be due to the arrangement of the primary and secondary bronchi, which, via their branching angles, generate inspiratory and expiratory aerodynamic valves. Both the anatomical similarity of the avian and alligator lung and the similarity in the patterns of airflow raise the possibility that these features are plesiomorphic for Archosauria and therefore did not evolve in response to selection for flapping flight or an endothermic metabolism, as has been generally assumed. To further test the hypothesis that unidirectional airflow is ancestral for Archosauria, we measured airflow in the lungs of the Nile crocodile (Crocodylus niloticus). As in birds and alligators, air flows cranially to caudally in the cervical ventral bronchus, and caudally to cranially in the dorsobronchi in the lungs of Nile crocodiles. We also visualized the gross anatomy of the primary, secondary and tertiary pulmonary bronchi of C. niloticus using computed tomography (CT) and microCT. The cervical ventral bronchus, cranial dorsobronchi and cranial medial bronchi display similar characteristics to their proposed homologues in the alligator, while there is considerable variation in the tertiary and caudal group bronchi. Our data indicate that the aspects of the crocodilian bronchial tree that maintain the aerodynamic valves and thus generate unidirectional airflow, are ancestral for Archosauria.
Keywords: Anatomy; Bronchi; Crocodylia; Endothermy; Evolution; Lung; Pneumaticity; Respiration.
Figures



References
-
- Bickler PE, Spragg RG, Hartman MT, White FN. Distribution of ventilation in American alligator, Alligator mississippiensis. American Journal of Physiology. 1985;249:477–481. - PubMed
-
- Boelert R. Sur la physiologie de la respirataion de l’ Alligator mississippiensis. Archives Internationales de Physiologie. 1942;52:57–72. doi: 10.3109/13813454209145577. - DOI
-
- Bonde N, Christiansen P. The detailed anatomy of Rhamphorhynchus: axial pneumaticity and its implications. London: Geological Society; 2003.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

