Comparison of subconjunctivally injected bevacizumab, ranibizumab, and pegaptanib for inhibition of corneal neovascularization in a rat model
- PMID: 23638411
- PMCID: PMC3633748
- DOI: 10.3980/j.issn.2222-3959.2013.02.05
Comparison of subconjunctivally injected bevacizumab, ranibizumab, and pegaptanib for inhibition of corneal neovascularization in a rat model
Abstract
Aim: To compare the efficacies of subconjunctival bevacizumab, ranibizumab, and pegaptanib sodium injections for the inhibition of corneal neovascularization in an experimental rat model.
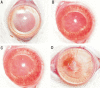
Methods: Sixteen corneas of 16 rats were chemically cauterized and randomized into four groups: bevacizumab group that treated with 0.05mL/1.25mg bevacizumab, ranibizumab group that treated with 0.05mL/0.5mg ranibizumab, pegaptanib group that treated with 0.05mL/0.15mg pegaptanib sodium, and control group that treated with 0.05mL saline solution. Digital photographs of the corneas were taken and analyzed using an image analysis software program. All corneas were excised and examined histologically on the 15(th) day.
Results: Each treatment group had significantly less neovascularized corneal areas and fewer blood vessels than the control group (all P<0.05). In addition, bevacizumab group had significantly less neovascularized corneal areas and fewer blood vessels than ranibizumab and pegaptanib groups (both P<0.05). However, there was no significant difference between the ranibizumab and pegaptanib groups regarding percentage of neovascularized corneal areas and number of blood vessels (both P>0.05).
Conclusion: Subconjunctival bevacizumab, ranibizumab, and pegaptanib sodium were effective with no corneal epitheliopathy for inhibiting corneal neovascularization after corneal burn in rats. Bevacizumab was more effective than ranibizumab and pegaptanib sodium.
Keywords: bevacizumab; corneal neovascularization; pegaptanib; ranibizumab; subconjunctival injection.
Figures

Similar articles
-
The impact of subconjuctivally injected EGF and VEGF inhibitors on experimental corneal neovascularization in rat model.Curr Eye Res. 2011 Nov;36(11):1005-13. doi: 10.3109/02713683.2011.601840. Curr Eye Res. 2011. PMID: 21999227
-
The effects of subconjunctival bevacizumab, ranibizumab, and aflibercept on corneal neovascularization.Hum Exp Toxicol. 2022 Jan-Dec;41:9603271221084674. doi: 10.1177/09603271221084674. Hum Exp Toxicol. 2022. PMID: 35465742
-
Comparison of the effects of bevacizumab and ranibizumab injection on corneal angiogenesis in an alkali burn induced model.Int J Ophthalmol. 2012;5(4):448-51. doi: 10.3980/j.issn.2222-3959.2012.04.08. Epub 2012 Aug 18. Int J Ophthalmol. 2012. PMID: 22937503 Free PMC article.
-
Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization.Saudi J Ophthalmol. 2017 Apr-Jun;31(2):99-105. doi: 10.1016/j.sjopt.2017.02.008. Epub 2017 Mar 8. Saudi J Ophthalmol. 2017. PMID: 28559722 Free PMC article. Review.
-
Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin).Ocul Surf. 2012 Apr;10(2):67-83. doi: 10.1016/j.jtos.2012.01.005. Epub 2012 Jan 25. Ocul Surf. 2012. PMID: 22482468 Free PMC article. Review.
Cited by
-
Comparing The Efficacy Of An Anti-Human VEGF-A Neutralizing Antibody Versus Bevacizumab On A Laser-Induced Choroidal Neovascularization (CNV) Rhesus Monkey Model.Drug Des Devel Ther. 2019 Nov 4;13:3813-3821. doi: 10.2147/DDDT.S219350. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31806932 Free PMC article.
-
Transcriptome Profiling of Neovascularized Corneas Reveals miR-204 as a Multi-target Biotherapy Deliverable by rAAVs.Mol Ther Nucleic Acids. 2018 Mar 2;10:349-360. doi: 10.1016/j.omtn.2017.12.019. Epub 2017 Dec 30. Mol Ther Nucleic Acids. 2018. PMID: 29499946 Free PMC article.
-
Management of corneal neovascularization: Current and emerging therapeutic approaches.Indian J Ophthalmol. 2024 May 1;72(Suppl 3):S354-S371. doi: 10.4103/IJO.IJO_3043_23. Epub 2024 Apr 20. Indian J Ophthalmol. 2024. PMID: 38648452 Free PMC article. Review.
-
Corneal neovascularization and biological therapy.J Med Life. 2015 Oct-Dec;8(4):444-8. J Med Life. 2015. PMID: 26664467 Free PMC article. Review.
-
Comparison of the Effects of Subconjunctival Injections of Bevacizumab and Interferon Alpha-2a on Corneal Angiogenesis in a Rat Model.Medicina (Kaunas). 2018 Apr 16;54(2):16. doi: 10.3390/medicina54020016. Medicina (Kaunas). 2018. PMID: 30344247 Free PMC article.
References
-
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(4):242–249. - PubMed
-
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43(3):245–269. - PubMed
-
- Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37(12):2485–494. - PubMed
-
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37(8):1625–1632. - PubMed
-
- Phillips GD, Stone AM, Jones BD, chultz JC, Whitehead RA, Knighton DR. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo. 1994;8(6):961–965. - PubMed
LinkOut - more resources
Full Text Sources
