Bevacizumab vs ranibizumab for neovascular age-related macular degeneration in Chinese patients
- PMID: 23638418
- PMCID: PMC3633755
- DOI: 10.3980/j.issn.2222-3959.2013.02.12
Bevacizumab vs ranibizumab for neovascular age-related macular degeneration in Chinese patients
Abstract
Aim: To compare the clinical efficacy of intravitreal injections of bevacizumab and ranibizumab for treating Chinese patients with neovascular age-related macular degeneration (AMD).
Methods: Among 60 Chinese patients with exudative AMD (60 eyes), 28 received intravitreal bevacizumab injections (1.25mg) and 32 received intravitreal ranibizumab injections (0.5mg), once a month for 3 months and were followed for a total of 6 months. Monthly optical coherence tomography (OCT) was used to determine whether the patients received additional treatments during the follow-up. We compared the baseline and 6-month follow-up values of mean best-corrected visual acuity (BCVA) and central retinal thickness (CRT) in both groups of patients. We also compared the occurrence of adverse events.
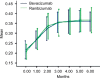
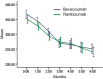
Results: At the 6-month follow-up, the mean BCVA (logMAR) of the bevacizumab and ranibizumab treatment groups improved from the baseline measurements of 0.72±0.23 and 0.73±0.22 to 0.47±0.14 and 0.45±0.20, respectively (P<0.05 for both groups). However, the change was not significantly different between the two groups. As evaluated by OCT, CRT decreased from 366.71±34.72µm and 352±36.9µm at baseline to 250.86±41.51µm and 243.22±41.38µm in the bevacizumab and ranibizumab groups, respectively (P<0.05 for both groups). However, the change was not significantly different between the two groups. There were no severe local adverse reactions or systemic adverse events.
Conclusion: Intravitreal bevacizumab and ranibizumab have equivalent effects on BCVA and CRT and appeare safe over the short-term.
Keywords: age-related macular degeneration; bevacizumab (avastin); choroidal neovascularization; ranibizumab (lucentis).
Figures
Similar articles
-
Comparative study of intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) in the treatment of neovascular age-related macular degeneration.Ophthalmologica. 2009;223(6):370-5. doi: 10.1159/000227783. Epub 2009 Jul 8. Ophthalmologica. 2009. PMID: 19590252
-
Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration.Indian J Ophthalmol. 2011 May-Jun;59(3):191-6. doi: 10.4103/0301-4738.81023. Indian J Ophthalmol. 2011. PMID: 21586838 Free PMC article. Clinical Trial.
-
Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab.Int J Retina Vitreous. 2021 Apr 1;7(1):26. doi: 10.1186/s40942-021-00299-4. Int J Retina Vitreous. 2021. PMID: 33795022 Free PMC article.
-
Intravitreal ranibizumab and bevacizumab therapy for choroidal neovascularization in age-related macular degeneration with extensive pre-existing geographic atrophy.Arq Bras Oftalmol. 2012 Jul-Aug;75(4):273-6. doi: 10.1590/s0004-27492012000400011. Arq Bras Oftalmol. 2012. PMID: 23258660
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration: a meta-analysis of randomized controlled trials.BMC Ophthalmol. 2018 May 30;18(1):130. doi: 10.1186/s12886-018-0785-3. BMC Ophthalmol. 2018. PMID: 29843663 Free PMC article.
Cited by
-
Decreased gray matter volume and increased white matter volume in patients with neovascular age-related macular degeneration: a voxel-based morphometry study.Aging (Albany NY). 2021 Oct 6;13(19):23182-23192. doi: 10.18632/aging.203610. Epub 2021 Oct 6. Aging (Albany NY). 2021. PMID: 34623972 Free PMC article.
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2019 Mar 4;3(3):CD005139. doi: 10.1002/14651858.CD005139.pub4. Cochrane Database Syst Rev. 2019. PMID: 30834517 Free PMC article.
-
Intravitreal Bevacizumab and Cardiovascular Risk in Patients with Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies.Drug Saf. 2016 Jun;39(6):517-41. doi: 10.1007/s40264-016-0408-y. Drug Saf. 2016. PMID: 26951234
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2014 Aug 29;8(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. Cochrane Database Syst Rev. 2014. PMID: 25170575 Free PMC article.
-
Understanding the Half-Life Extension of Intravitreally Administered Antibodies Binding to Ocular Albumin.Pharmaceutics. 2020 Aug 26;12(9):810. doi: 10.3390/pharmaceutics12090810. Pharmaceutics. 2020. PMID: 32858986 Free PMC article.
References
-
- Zou HD, Zhang X, Xu X, Wang FH, Zhang SJ. Age-related macular degeneration prevalence survey of Caojiadu street Jing'an district in Shanghai. ZhonghuaYanke Zhazhi. 2005;41(1):15–19. - PubMed
-
- Lou ZL, Chen G, Tang R. Age-related macular degeneration prevalence survey of agency personnel in Changsha city. Ophthalmol Res. 2008;26(11):822–823.
-
- Ni Z. 1st edition. Shanghai: Shanghai Scientific Popularization Publishing House; 2002. Pathological anatomy and clinical of ophthalmology; pp. 281–282.
-
- Sheng YJ. Advances in the treatment of age-related macular degeneration. J Clin Ophthalmol. 2004;12(5):471.
-
- Bressler NM. Antiangiogenic approaches to aged-related macular degeneration today. Ophthalmology. 2009;116(10 Suppl):S15–23. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials